J Dent Anesth Pain Med.
2016 Mar;16(1):39-47. 10.17245/jdapm.2016.16.1.39.
Effects of propofol-induced autophagy against oxidative stress in human osteoblasts
- Affiliations
-
- 1Department of Dental Anesthesia and Pain Medicine, School of Dentistry, Pusan National University, Dental Research Institute, Gyeongnam, Korea. anekch@pusan.ac.kr
- 2Department of Oral Anatomy, School of Dentistry, Pusan National University, Gyeongnam, Korea.
- 3Department of Anesthesia and Pain Medicine, School of Medicine, Pusan National University, Gyeongnam, Korea.
- KMID: 2308764
- DOI: http://doi.org/10.17245/jdapm.2016.16.1.39
Abstract
- BACKGROUND
Oxidative stress occurs during the aging process and other conditions such as bone fracture, bone diseases, and osteoporosis, but the role of oxidative stress in bone remodeling is unknown. Propofol exerts antioxidant effects, but the mechanisms of propofol preconditioning on oxidative stress have not been fully explained. Therefore, the aim of this study was to evaluate the protective effects of propofol against H2O2-induced oxidative stress on a human fetal osteoblast (hFOB) cell line via activation of autophagy.
METHODS
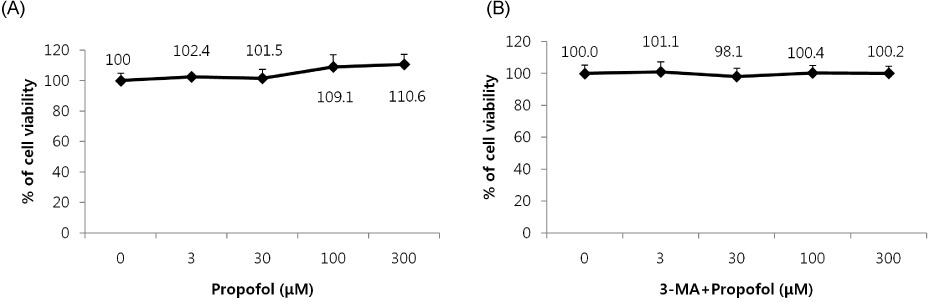
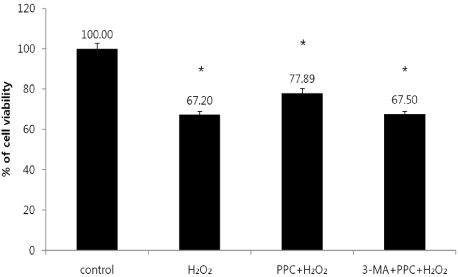
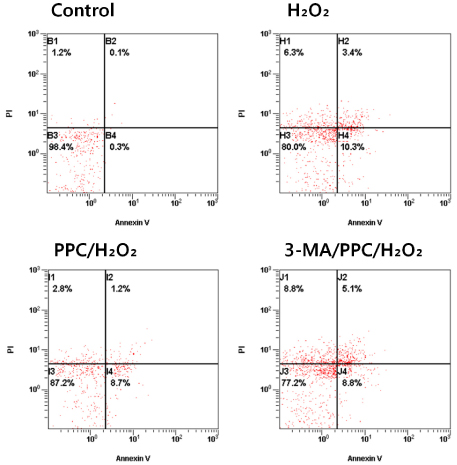
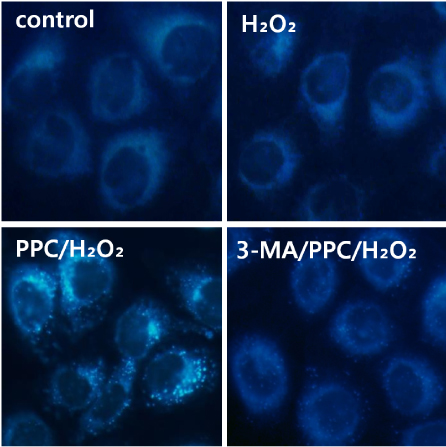
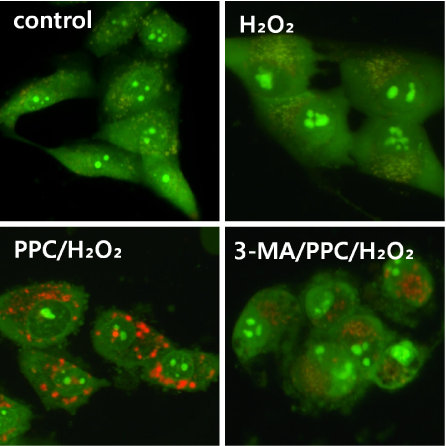
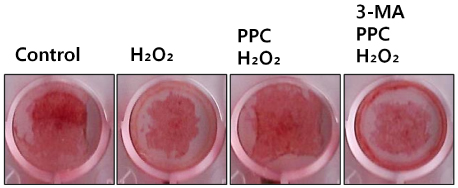
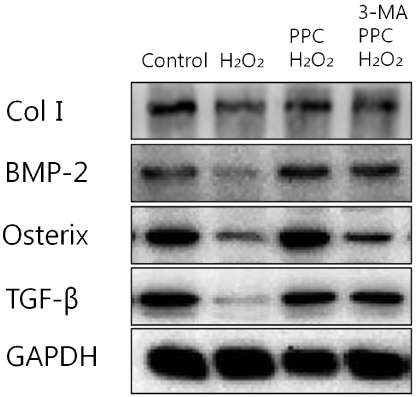
Cells were randomly divided into the following groups: control cells were incubated in normoxia (5% CO2, 21% O2, and 74% N2) without propofol. Hydrogen peroxide (H2O2) group cells were exposed to H2O2 (200 µM) for 2 h, propofol preconditioning (PPC)/H2O2 group cells were pretreated with propofol then exposed to H2O2, 3-methyladenine (3-MA)/PPC/H2O2 cells were pretreated with 3-MA (1 mM) and propofol, then were exposed to H2O2. Cell viability and apoptosis were evaluated. Osteoblast maturation was determined by assaying bone nodular mineralization. Expression levels of bone related proteins were determined by western blot.
RESULTS
Cell viability and bone nodular mineralization were decreased significantly by H2O2, and this effect was rescued by propofol preconditioning. Propofol preconditioning effectively decreased H2O2-induced hFOB cell apoptosis. However, pretreatment with 3-MA inhibited the protective effect of propofol. In western blot analysis, propofol preconditioning increased protein levels of collagen type I, BMP-2, osterix, and TGF-β1.
CONCLUSIONS
This study suggests that propofol preconditioning has a protective effect on H2O2-induced hFOB cell death, which is mediated by autophagy activation.
Keyword
MeSH Terms
Figure
Reference
-
1. Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000; 289:1504–1508.
Article2. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003; 423:337–342.
Article3. Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev. 2000; 21:393–411.
Article4. Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, et al. Novel regulators of bone formation: Molecular clones and activities. Science. 1988; 242:1528–1534.
Article5. Xu ZS, Wang XY, Xiao DM, Hu LF, Lu M, Wu ZY, et al. Hydrogen sulfide protects MC3T3-E1 osteoblastic cells against H2O2-induced oxidative damage-implications for the treatment of osteoporosis. Free Radic Biol Med. 2011; 50:1314–1323.
Article6. Lean JM, Jagger CJ, Kirstein B, Fuller K, Chambers TJ. Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology. 2005; 146:728–735.
Article7. Panieri E, Gogvadze V, Norberg E, Venkatesh R, Orrenius S, Zhivotovsky B. Reactive oxygen species generated in different compartments induce cell death, survival, or senescence. Free Radic Biol Med. 2013; 57:176–187.
Article8. Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta. 2006; 1758:994–1003.
Article9. Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, et al. ROS signaling: The new wave? Trends Plant Sci. 2011; 16:300–309.
Article10. Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: A key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000; 2000:pe1.
Article11. Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011; 194:7–15.
Article12. Zhu Y, Park SH, Ozden O, Kim HS, Jiang H, Vassilopoulos A, et al. Exploring the electrostatic repulsion model in the role of Sirt3 in directing MnSOD acetylation status and enzymatic activity. Free Radic Biol Med. 2012; 53:828–833.
Article13. Eno CO, Zhao G, Venkatanarayan A, Wang B, Flores ER, Li C. Noxa couples lysosomal membrane permeabilization and apoptosis during oxidative stress. Free Radic Biol Med. 2013; 65:26–37.
Article14. Kim YN, Jung HY, Eum WS, Kim DW, Shin MJ, Ahn EH, et al. Neuroprotective effects of PEP-1-carbonyl reductase 1 against oxidative-stress-induced ischemic neuronal cell damage. Free Radic Biol Med. 2014; 69:181–196.
Article15. Ito H, Watanabe Y, Isshiki A, Uchino H. Neuroprotective properties of propofol and midazolam, but not pentobarbital, on neuronal damage induced by forebrain ischemia, based on the GABAA receptors. Acta Anaesthesiol Scand. 1999; 43:153–162.
Article16. Yano T, Nakayama R, Ushijima K. Intracerebroventricular propofol is neuroprotective against transient global ischemia in rats: Extracellular glutamate level is not a major determinant. Brain Res. 2000; 883:69–76.
Article17. Bayona NA, Gelb AW, Jiang Z, Wilson JX, Urquhart BL, Cechetto DF. Propofol neuroprotection in cerebral ischemia and its effects on low-molecular-weight antioxidants and skilled motor tasks. Anesthesiology. 2004; 100:1151–1159.
Article18. Wu GJ, Chen WF, Hung HC, Jean YH, Sung CS, Chakraborty C, et al. Effects of propofol on proliferation and anti-apoptosis of neuroblastoma SH-SY5Y cell line: New insights into neuroprotection. Brain Res. 2011; 1384:42–50.
Article19. Gulcin I, Alici HA, Cesur M. Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem Pharm Bull (Tokyo). 2005; 53:281–285.
Article20. Schultz JE, Gross GJ. Opioids and cardioprotection. Pharmacol Ther. 2001; 89:123–137.
Article21. Tanaka K, Ludwig LM, Kersten JR, Pagel PS, Warltier DC. Mechanisms of cardioprotection by volatile anesthetics. Anesthesiology. 2004; 100:707–721.
Article22. Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina D, Settembre C, et al. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat Cell Biol. 2010; 12:863–875.
Article23. Mizushima N. The pleiotropic role of autophagy: From protein metabolism to bactericide. Cell Death Differ. 2005; 12:Suppl 2. 1535–1541.
Article24. Chen W, Sun Y, Liu K, Sun X. Autophagy: A double-edged sword for neuronal survival after cerebral ischemia. Neural Regen Res. 2014; 9:1210–1216.
Article25. Schelonka EP, Usher A. Ipriflavone and osteoporosis. JAMA. 2001; 286:1836–1837.
Article26. Myers G, Prince RL, Kerr DA, Devine A, Woodman RJ, Lewis JR, et al. Tea and flavonoid intake predict osteoporotic fracture risk in elderly australian women: A prospective study. Am J Clin Nutr. 2015; 102:958–965.
Article27. Gerstenfeld LC, Thiede M, Seibert K, Mielke C, Phippard D, Svagr B, et al. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003; 21:670–675.
Article28. Li X, Cao X. BMP signaling and skeletogenesis. Ann N Y Acad Sci. 2006; 1068:26–40.
Article29. Noda M, Camilliere JJ. In vivo stimulation of bone formation by transforming growth factor-beta. Endocrinology. 1989; 124:2991–2994.
Article30. Pavlin D, Zadro R, Gluhak-Heinrich J. Temporal pattern of stimulation of osteoblast-associated genes during mechanically-induced osteogenesis in vivo: Early responses of osteocalcin and type I collagen. Connect Tissue Res. 2001; 42:135–148.
Article31. Ryoo HM, Hoffmann HM, Beumer T, Frenkel B, Towler DA, Stein GS, et al. Stage-specific expression of dlx-5 during osteoblast differentiation: Involvement in regulation of osteocalcin gene expression. Mol Endocrinol. 1997; 11:1681–1694.
Article32. Jagodzinski M, Drescher M, Zeichen J, Hankemeier S, Krettek C, Bosch U, et al. Effects of cyclic longitudinal mechanical strain and dexamethasone on osteogenic differentiation of human bone marrow stromal cells. Eur Cell Mater. 2004; 7:35. 41. discussion 41.
Article33. Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996; 382:448–452.
Article34. Chen Y, Gibson SB. Is mitochondrial generation of reactive oxygen species a trigger for autophagy? Autophagy. 2008; 4:246–248.
Article35. Baehrecke EH. Autophagy: Dual roles in life and death. Nat Rev Mol Cell Biol. 2005; 6:505–510.
Article36. Levine B, Yuan J. Autophagy in cell death: An innocent convict? J Clin Invest. 2005; 115:2679–2688.
Article37. Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005; 5:726–734.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Propofol protects human keratinocytes from oxidative stress via autophagy expression
- Propofol protects against oxidative-stress-induced COS-7 cell apoptosis by inducing autophagy
- Protective effects of remifentanil against Hâ‚‚Oâ‚‚-induced oxidative stress in human osteoblasts
- Effects of Antioxidant on Oxidative Stress and Autophagy in Bronchial Epithelial Cells Exposed to Particulate Matter and Cigarette Smoke Extract
- Phelligridin D maintains the function of periodontal ligament cells through autophagy in glucose-induced oxidative stress