J Dent Anesth Pain Med.
2016 Dec;16(4):263-271. 10.17245/jdapm.2016.16.4.263.
Protective effects of remifentanil against Hâ‚‚Oâ‚‚-induced oxidative stress in human osteoblasts
- Affiliations
-
- 1Department of Dental Anesthesia and Pain Medicine, School of Dentistry, Pusan National University, Dental Research Institute, Yangsan, Republic of Korea. myeong-clinic@daum.net hjoonkim@pusan.ac.kr
- 2Department of Oral Anatomy, School of Dentistry, Pusan National University, Yangsan, Republic of Korea.
- 3Department of Anesthesia and Pain Medicine, School of Medicine, Pusan National University, Yangsan, Republic of Korea.
- 4Department of Oral Physiology, School of Dentistry, Pusan National University, Yangsan, Republic of Korea. myeong-clinic@daum.net hjoonkim@pusan.ac.kr
- KMID: 2366154
- DOI: http://doi.org/10.17245/jdapm.2016.16.4.263
Abstract
- BACKGROUND
Bone injury is common in many clinical situations, such as surgery or trauma. During surgery, excessive reactive oxygen species (ROS) production decreases the quality and quantity of osteoblasts. Remifentanil decreases ROS production, reducing oxidative stress and the inflammatory response. We investigated remifentanil's protective effects against Hâ‚‚Oâ‚‚-induced oxidative stress in osteoblasts.
METHODS
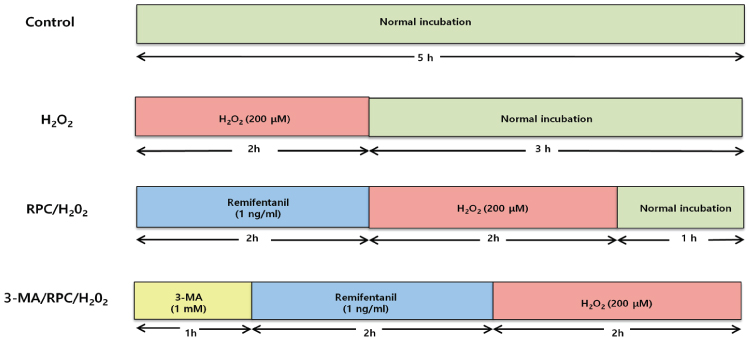
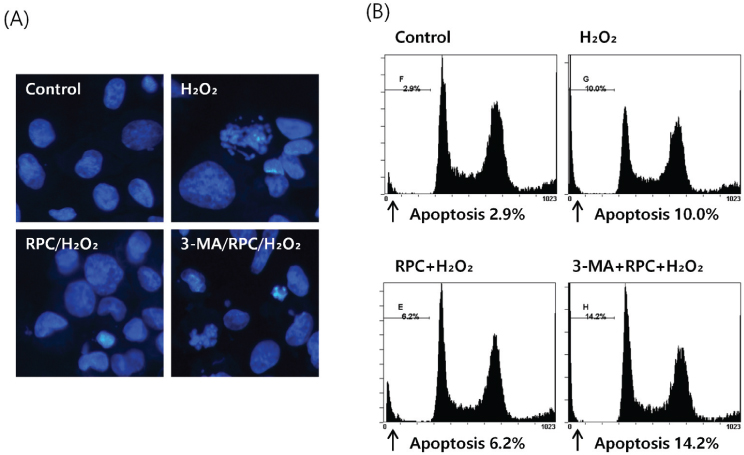
To investigate the effect of remifentanil on human fetal osteoblast (hFOB) cells, the cells were incubated with 1 ng/ml of remifentanil for 2 h before exposure to H2O2. For induction of oxidative stress, hFOB cells were then treated with 200 µM Hâ‚‚Oâ‚‚ for 2 h. To evaluate the effect on autophagy, a separate group of cells were incubated with 1 mM 3-methyladenine (3-MA) before treatment with remifentanil and Hâ‚‚Oâ‚‚. Cell viability and apoptotic cell death were determined via MTT assay and Hoechst staining, respectively. Mineralized matrix formation was visualized using alizarin red S staining. Western blot analysis was used to determine the expression levels of bone-related genes.
RESULTS
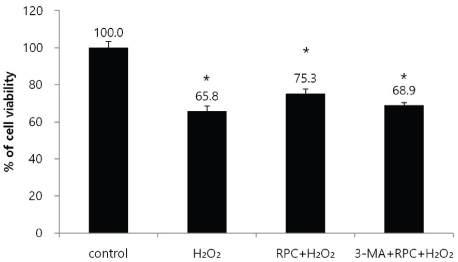
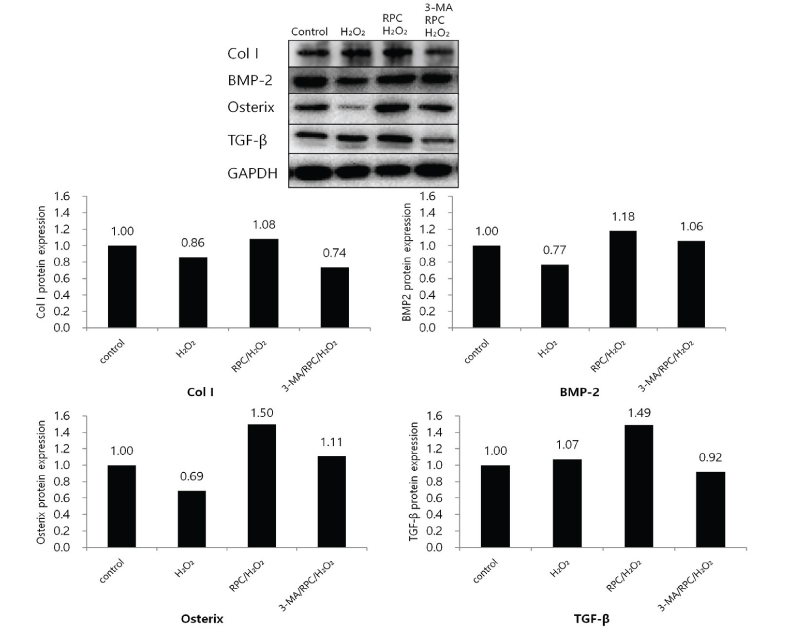
Cell viability and mineralized matrix formation increased on remifentanil pretreatment before exposure to H₂O₂-induced oxidative stress. As determined via western blot analysis, remifentanil pretreatment increased the expression of bone-related genes (Col I, BMP-2, osterix, and TGF-β). However , pretreatment with 3-MA before exposure to remifentanil and H₂O₂ inhibited remifentanil's protective effects on hFOB cells during oxidative stress.
CONCLUSIONS
We showed that remifentanil prevents oxidative damage in hFOB cells via a mechanism that may be highly related to autophagy. Further clinical studies are required to investigate its potential as a therapeutic agent.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Effect of remifentanil on pre-osteoclast cell differentiation in vitro
Hyun-Ook Jeon, In-Seok Choi, Ji-Young Yoon, Eun-Jung Kim, Ji-Uk Yoon, Ah-Reum Cho, Hyung-Joon Kim, Cheul-Hong Kim
J Dent Anesth Pain Med. 2018;18(1):9-17. doi: 10.17245/jdapm.2018.18.1.9.Remifentanil promotes osteoblastogenesis by upregulating Runx2/osterix expression in preosteoblastic C2C12 cells
Ji-Young Yoon, Tae-Sung Kim, Ji-Hye Ahn, Ji-Uk Yoon, Hyung-Joon Kim, Eun-Jung Kim
J Dent Anesth Pain Med. 2019;19(2):91-99. doi: 10.17245/jdapm.2019.19.2.91.
Reference
-
1. Masi L, Brandi ML. Physiopathological basis of bone turnover. Q J Nucl Med. 2001; 45:2–6.
Article2. Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011; 377:1276–1287.
Article3. Seeman E. Invited Review: Pathogenesis of osteoporosis. J Appl Physiol. 2003; 95:2142–2151.
Article4. Polzer K, Joosten L, Gasser J, Distler JH, Ruiz G, Baum W, et al. Interleukin-1 is essential for systemic inflammatory bone loss. Ann Rheum Dis. 2010; 69:284–290.
Article5. Shen CL, Yeh JK, Samathanam C, Cao JJ, Stoecker BJ, Dagda RY, et al. Protective actions of green tea polyphenols and alfacalcidol on bone microstructure in female rats with chronic inflammation. J Nutr Biochem. 2011; 22:673–680.
Article6. Arai M, Shibata Y, Pugdee K, Abiko Y, Ogata Y. Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life. 2007; 59:27–33.
Article7. Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001; 31:509–519.
Article8. Gasbarrini A, Grigolo B, Serra M, Baldini N, Scotlandi K, Gasbarrini A, et al. Generation of free radicals during anoxia and reoxygenation in perfused osteoblastlike cells. Clin Orthop Relat Res. 1997; (338):247–252.
Article9. Chae HZ, Kang SW, Rhee SG. Isoforms of mammalian peroxiredoxin that reduce peroxides in presence of thioredoxin. Methods Enzymol. 1999; 300:219–226.
Article10. Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012; 24:981–990.
Article11. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008; 451:1069–1075.
Article12. Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015; 22:377–388.
Article13. Filomeni G, Desideri E, Cardaci S, Rotilio G, Ciriolo MR. Under the ROS: Thiol network is the principal suspect for autophagy commitment. Autophagy. 2010; 6:999–1005.
Article14. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009; 417:1–13.
Article15. Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007; 14:146–157.
Article16. Komatsu R, Turan AM, Orhan-Sungur M, McGuire J, Radke OC, Apfel CC. Remifentanil for general anaesthesia: a systematic review. Anaesthesia. 2007; 62:1266–1280.
Article17. Yang LQ, Tao KM, Liu YT, Cheung CW, Irwin MG, Wong GT, et al. Remifentanil preconditioning reduces hepatic ischemia-reperfusion injury in rats via inducible nitric oxide synthase expression. Anesthesiology. 2011; 114:1036–1047.
Article18. Zongze Z, Jia Z, Chang C, Kai C, Yanlin W. Protective effects of remifentanil on septic mice. Mol Biol Rep. 2010; 37:2803–2808.
Article19. Baik SW, Park BS, Kim YH, Kim YD, Kim CH, Yoon JY, et al. Effects of Remifentanil Preconditioning on Osteoblasts under Hypoxia-Reoxygenation Condition. Int J Med Sci. 2015; 12:583–589.20. Imlay JA, Linn S. DNA damage and oxygen radical toxicity. Science. 1988; 240:1302–1309.21. Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003; 17:1195–1214.
Article22. Cadet J, Delatour T, Douki T, Gasparutto D, Pouget JP, Ravanat JL, et al. Hydroxyl radicals and DNA base damage. Mutat Res. 1999; 424:9–21.
Article23. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000; 408:239–247.
Article24. Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, et al. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun. 2004; 314:197–207.
Article25. Baek KH, Oh KW, Lee WY, Lee SS, Kim MK, Kwon HS, et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif Tissue Int. 2010; 87:226–235.
Article26. Li X, Cao X. BMP signaling and skeletogenesis. Ann N Y Acad Sci. 2006; 1068:26–40.
Article27. Kanonidou Z, Karystianou G. Anesthesia for the elderly. Hippokratia. 2007; 11:175–177.
Article28. Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002; 359:1761–1767.
Article29. Pavlin D, Zadro R, Gluhak-Heinrich J. Temporal pattern of stimulation of osteoblast-associated genes during mechanically-induced osteogenesis in vivo: early responses of osteocalcin and type I collagen. Connect Tissue Res. 2001; 42:135–148.
Article30. Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996; 382:448–452.
Article31. Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996; 382:448–452.
Article32. Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004; 22:233–241.
Article33. Huang Q, Wu YT, Tan HL, Ong CN, Shen HM. A novel function of poly(ADP-ribose) polymerase-1 in modulation of autophagy and necrosis under oxidative stress. Cell Death Differ. 2009; 16:264–277.
Article34. Gozuacik D, Kimchi A. Autophagy and cell death. Curr Top Dev Biol. 2007; 78:217–245.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Remifentanil Preconditioning Attenuating Oxidative Stress in Human Dermal Fibroblast
- Remifentanil induces autophagy and prevents hydrogen peroxide-induced apoptosis in Cos-7 cells
- Dexmedetomidine attenuates Hâ‚‚Oâ‚‚-induced cell death in human osteoblasts
- Inhibition of Store-Operated Calcium Entry Protects Endothelial Progenitor Cells from Hâ‚‚Oâ‚‚-Induced Apoptosis
- Cytoprotective Effect of Taurine against Hydrogen Peroxide-Induced Oxidative Stress in UMR-106 Cells through the Wnt/β-Catenin Signaling Pathway