J Dent Anesth Pain Med.
2016 Dec;16(4):295-302. 10.17245/jdapm.2016.16.4.295.
Dexmedetomidine attenuates Hâ‚‚Oâ‚‚-induced cell death in human osteoblasts
- Affiliations
-
- 1Department of Dental Anesthesia and Pain Medicine, School of Dentistry, Pusan National University, Dental Research Institute, Yangsan, Republic of Korea. ddskdw@naver.com
- 2Department of Oral Anatomy, School of Dentistry, Pusan National University, Yangsan, Republic of Korea.
- 3Department of Anesthesia and Pain Medicine, School of Medicine, Pusan National University, Yangsan, Republic of Korea. shinsw@pusan.ac.kr
- KMID: 2366158
- DOI: http://doi.org/10.17245/jdapm.2016.16.4.295
Abstract
- BACKGROUND
Reactive oxygen species play critical roles in homeostasis and cell signaling. Dexmedetomidine, a specific agonist of the α₂-adrenoceptor, has been commonly used for sedation, and it has been reported to have a protective effect against oxidative stress. In this study, we investigated whether dexmedetomidine has a protective effect against H₂O₂-induced oxidative stress and the mechanism of H₂O₂-induced cell death in normal human fetal osteoblast (hFOB) cells.
METHODS
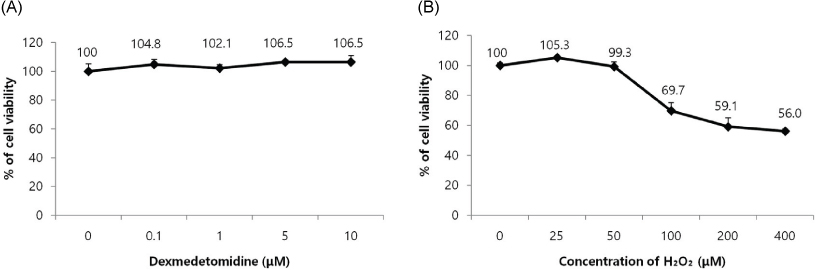
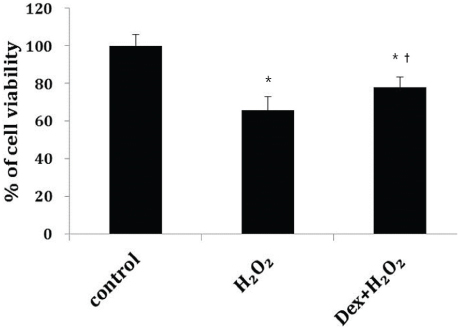
Cells were divided into three groups: control group"”cells were incubated in normoxia without dexmedetomidine, hydrogen peroxide (H2O2) group"”cells were exposed to Hâ‚‚Oâ‚‚ (200 µM) for 2 h, and Dex/Hâ‚‚Oâ‚‚ group"”cells were pretreated with dexmedetomidine (5 µM) for 2 h then exposed to Hâ‚‚Oâ‚‚ (200 µM) for 2 h. Cell viability and apoptosis were evaluated. Osteoblast maturation was determined by assaying bone nodular mineralization. Expression levels of bone-related proteins were determined by western blot.
RESULTS
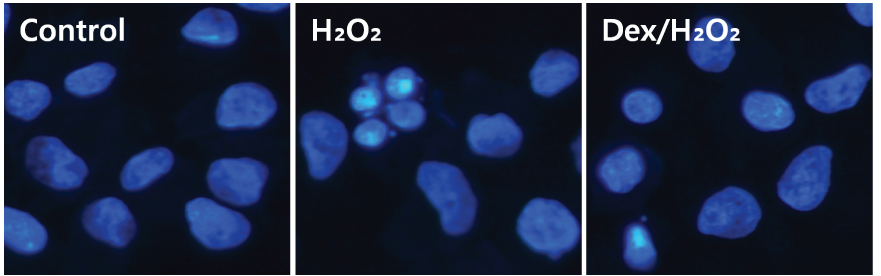
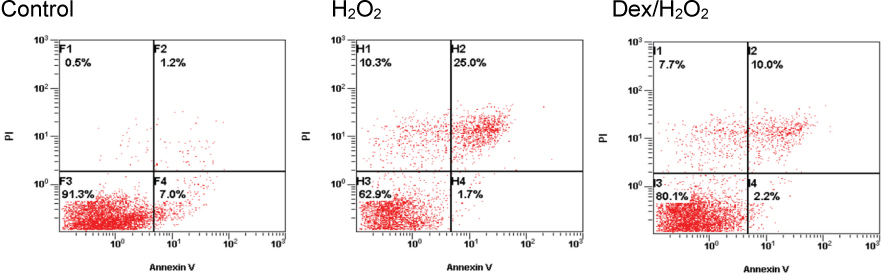
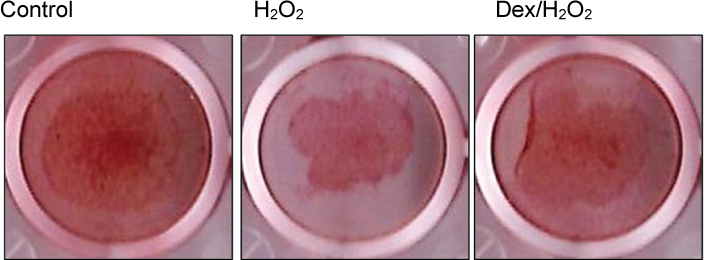
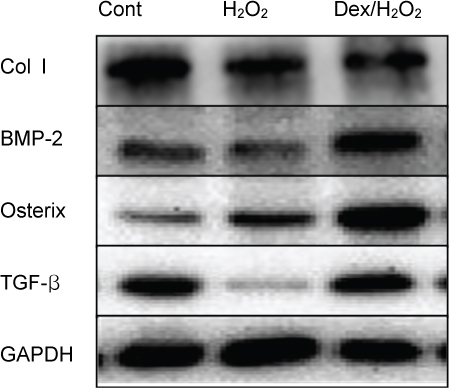
Cell viability was significantly decreased in the Hâ‚‚Oâ‚‚ group compared with the control group, and this effect was improved by dexmedetomidine. The Hoechst 33342 and Annexin-V FITC/PI staining revealed that dexmedetomidine effectively decreased Hâ‚‚Oâ‚‚-induced hFOB cell apoptosis. Dexmedetomidine enhanced the mineralization of hFOB cells when compared to the Hâ‚‚Oâ‚‚ group. In western blot analysis, bone-related protein was increased in the Dex/Hâ‚‚Oâ‚‚ group.
CONCLUSIONS
We demonstrated the potential therapeutic value of dexmedetomidine in Hâ‚‚Oâ‚‚-induced oxidative stress by inhibiting apoptosis and enhancing osteoblast activity. Additionally, the current investigation could be evidence to support the antioxidant potential of dexmedetomidine in vitro.
Keyword
MeSH Terms
Figure
Reference
-
1. Lee D, Kook SH, Ji H, Lee SA, Choi KC, Lee KY, et al. N-acetyl cysteine inhibits H2O2-mediated reduction in the mineralization of MC3T3-E1 cell by down-regulating Nrf2/HO-1 pathway. BMB Rep. 2015; 48:636–641.
Article2. Song Q, Gou WL, Zhang R. FAM3A Protects HT22 Cells Against Hydrogen Peroxide-Induced Oxidative Stress Through Activation of PI3K/Akt but not MEK/ERK Pathway. Cell Physiol Biochem. 2015; 37:1431–1441.
Article3. Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci. 2006; 1092:385–396.
Article4. Hapidin H, Rozelan D, Abdullah H, Wan Hanaffi WN, Soelaiman IN. Quercus infectoria gall extract enhanced the proliferation and activity of human fetal osteoblast cell line (hFOB 1.19). Malays J Med Sci. 2015; 22:12–22.5. Wang F, Yin P, Lu Y, Zhou Z, Jiang C, Liu Y, et al. Cordycepin prevents oxidative stress-induced inhibition of osteogenesis. Oncotarget. 2015; 6:35496–35508.
Article6. Cui J, Zhao H, Yi B, Zeng J, Lu K, Ma D. Dexmedetomidine attenuates bilirubin-induced lung alveolar epithelial cell death in vitro and in vivo. Crit Care Med. 2015; 43:e356–e368.
Article7. Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bayl Univ Med Cent). 2001; 14:13–21.
Article8. Kip G, Celik A, Bilge M, Alkan M, Kiraz HA, Ozer A, et al. Dexmedetomidine protects from post myocardial ischaemia reperfusion lung damage in diabetic rats. Libyan J Med. 2015; 10:27828.
Article9. Zhang XK, Zhou XP, Zhang Q, Zhu F. The preventive effects of dexmedetomidine against intestinal ischemia-reperfusion injury in Wistar rats. Iran J Basic Med Sci. 2015; 18:604–609.10. Sezer A, Memis D, Usta U, Sut N. The effect of dexmedetomidine on liver histopathology in a rat sepsis model: an experimental pilot study. Ulus Travma Acil Cerrahi Derg. 2010; 16:108–112.11. Kocoglu H, Ozturk H, Ozturk H, Yilmaz F, Gulcu N. Effect of dexmedetomidine on ischemia-reperfusion injury in rat kidney: a histopathologic study. Ren Fail. 2009; 31:70–74.
Article12. Huang CX, Lv B, Wang Y. Protein phosphatase 2A mediates oxidative stress induced apoptosis in osteoblasts. Mediators Inflamm. 2015; 2015:804260.
Article13. Wang L, Zhang YG, Wang XM, Ma LF, Zhang YM. Naringin protects human adipose-derived mesenchymal stem cells against hydrogen peroxide-induced inhibition of osteogenic differentiation. Chem Biol Interact. 2015; 242:255–261.
Article14. Wang G, Liu C, Liu J, Liu B, Li P, Qin G, et al. Exopolysaccharide from trichoderma pseudokoningii induces the apoptosis of MCF-7 cells through an intrinsic mitochondrial pathway. Carbohydr Polym. 2016; 136:1065–1073.
Article15. Jung IL, Lee JH, Kang SC. A potential oral anticancer drug candidate, leaf extract, induces the apoptosis of human hepatocellular carcinoma cells. Oncol Lett. 2015; 10:1597–1604.
Article16. Rieger AM, Nelson KL, Konowalchuk JD, Barreda DR. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J Vis Exp. 2011; 50:e2597.
Article17. van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelinqsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998; 31:1–9.
Article18. Wilkins RC, Kutzner BC, Truong M, Sanchez-Dardon J, McLean JR. Analysis of radiation-induced apoptosis in human lymphocytes: Flow cytometry using Annexin V and propidium iodide versus the neutral comet assay. Cytometry. 2002; 48:14–19.
Article19. Sawai H, Domae N. Discrimination between primary necrosis and apoptosis by necrostatin-1 in Annexin V positive/propidium iodide-negative cells. Biochem Biophys Res Commun. 2011; 411:569–573.
Article20. Span LF, Pennings AH, Vierwinden G, Boezeman JB, Raymakers RA, de Witte T. The dynamic process of apoptosis analyzed by flow cytometry using Annexin-V/propidium iodide and a modified in situ end labeling technique. Cytometry. 2002; 47:24–31.
Article21. Ren H, Ren H, Li X, Yu D, Mu S, Chen Z, et al. Effects of intermedin on proliferation, apoptosis and the expression of OPG/RANKL/M-CSF in the MC3T3-E1 osteoblast cell line. Mol Med Rep. 2015; 12:6711–6717.
Article22. Xiong XC, Zhu Y, Ge R, Liu LF, Yuan W. Effect of Melatonin on the extracellular-regulated kinase signal pathway activation and human osteoblastic cell line hFOB 1.19 proliferation. Int J Mol Sci. 2015; 16:10337–10353.
Article23. Jiang T, Zhou B, Huang L, Wu H, Huang J, Liang T, et al. Andrographolide exerts pro-osteogenic effect by activation of Wnt/β-Catenin signaling pathway in vitro. Cell Physiol Biochem. 2015; 36:2327–2339.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective effects of remifentanil against Hâ‚‚Oâ‚‚-induced oxidative stress in human osteoblasts
- Propofol protects human keratinocytes from oxidative stress via autophagy expression
- Cytoprotective Mechanism of Cyanidin and Delphinidin against Oxidative Stress-Induced Tenofibroblast Death
- Oleanolic acid induced autophagic cell death in hepatocellular carcinoma cells via PI3K/Akt/mTOR and ROS-dependent pathway
- Hydrogen peroxide attenuates refilling of intracellular calcium store in mouse pancreatic acinar cells