Korean J Physiol Pharmacol.
2016 May;20(3):237-243. 10.4196/kjpp.2016.20.3.237.
Oleanolic acid induced autophagic cell death in hepatocellular carcinoma cells via PI3K/Akt/mTOR and ROS-dependent pathway
- Affiliations
-
- 1Department of General Surgery, Affiliated Hospital of Xuzhou Medical College, Xuzhou, Jiangsu 221002, P.R. China.
- 2Department of Basic Medical, Xuzhou Medical College, Xuzhou, Jiangsu 221002, P.R. China. tengdc2012@163.com
- KMID: 2285595
- DOI: http://doi.org/10.4196/kjpp.2016.20.3.237
Abstract
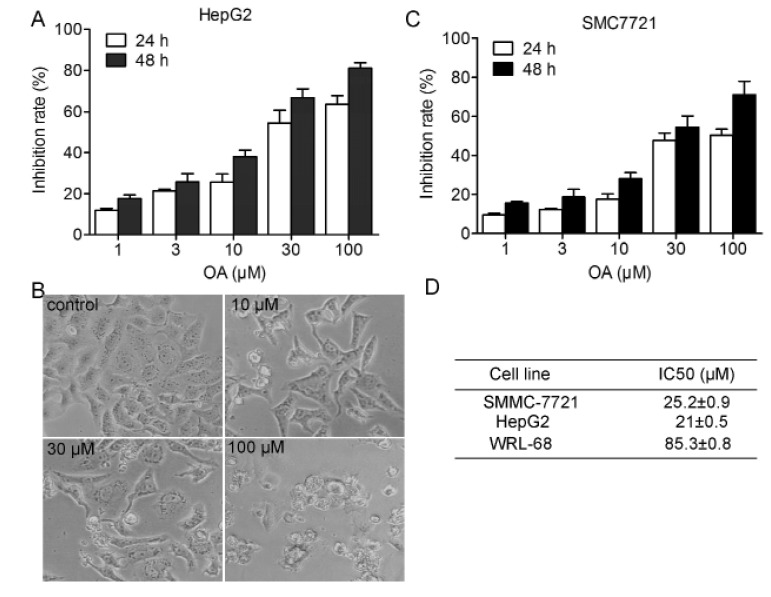
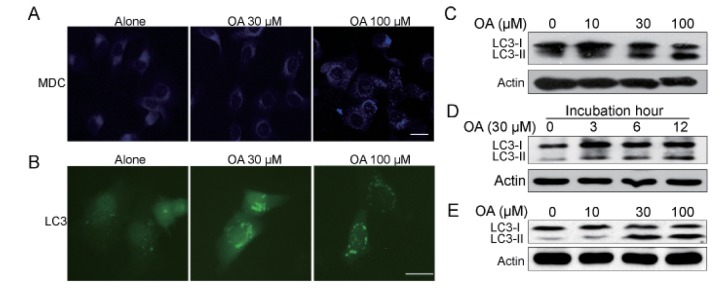
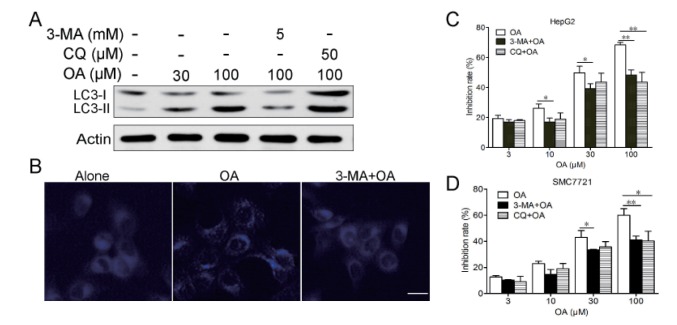
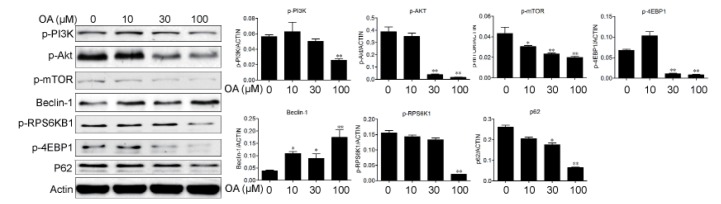
- Oleanolic acid (OA) has a wide variety of bioactivities such as hepatoprotective, anti-inflammatory and anti-cancer activity and is used for medicinal purposes in many Asian countries. In the present study, the effect of OA on induction of autophagy in human hepatocellular carcinoma HepG2 and SMC7721 cells and the related mechanisms were investigated. MTT assay showed that OA significantly inhibited HepG2 and SMC7721 cells growth. OA treatment enhanced formation of autophagic vacuoles as revealed by monodansylcadaverine (MDC) staining. At the same time, increasing punctuate distribution of microtubule-associated protein 1 light chain 3 (LC3) and an increasing ratio of LC3-II to LC3-I were also triggered by OA incubation. In addition, OA-induced cell death was signifi cantly inhibited by autophagy inhibitors 3-methyladenine (3-MA) and chloroquine (CQ) pretreatment. And we found out that OA can suppress the PI3K/Akt1/mTOR signaling pathway. Furthermore, our data suggested that OA-triggered autophagy was ROS-dependent as demonstrated by elevated cellular ROS levels by OA treatment. When ROS was cleared by N-acetylcysteine (NAC), OA-induced LC3-II convertsion and cell death were all reversed. Taken together, our results suggest that OA exerts anticancer eff ect via autophagic cell death in hepatocellular carcinoma.
Keyword
MeSH Terms
Figure
Reference
-
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127:2893–2917. PMID: 21351269.
Article2. Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208–1236. PMID: 16250051.
Article3. Carr BI. Hepatocellular carcinoma: current management and future trends. Gastroenterology. 2004; 127(5 Suppl 1):S218–S224. PMID: 15508087.
Article4. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007; 132:2557–2576. PMID: 17570226.
Article5. Singh GB, Singh S, Bani S, Gupta BD, Banerjee SK. Anti- inflammatory activity of oleanolic acid in rats and mice. J Pharm Pharmacol. 1992; 44:456–458. PMID: 1359067.6. Mengoni F, Lichtner M, Battinelli L, Marzi M, Mastroianni CM, Vullo V, Mazzanti G. In vitro anti-HIV activity of oleanolic acid on infected human mononuclear cells. Planta Med. 2002; 68:111–114. PMID: 11859458.
Article7. Bian Q, Liu SF, Huang JH, Yang Z, Tang DZ, Zhou Q, Ning Y, Zhao YJ, Lu S, Shen ZY, Wang YJ. Oleanolic acid exerts an osteoprotective effect in ovariectomy-induced osteoporotic rats and stimulates the osteoblastic differentiation of bone mesenchymal stem cells in vitro. Menopause. 2012; 19:225–233. PMID: 22011754.
Article8. Liu J, Lu YF, Zhang Y, Wu KC, Fan F, Klaassen CD. Oleanolic acid alters bile acid metabolism and produces cholestatic liver injury in mice. Toxicol Appl Pharmacol. 2013; 272:816–824. PMID: 23948738.
Article9. Wan X, Liu J, Lu YF. Hepatoprotective effect of oleanolic acid on D-galactosamine-induced acute liver injury in mice. Acta Pharmacol Sin. 2013; 34(Suppl 1):30–31.10. Guo G, Yao W, Zhang Q, Bo Y. Oleanolic acid suppresses migration and invasion of malignant glioma cells by inactivating MAPK/ERK signaling pathway. PLoS One. 2013; 8:e72079. PMID: 23991044.
Article11. Li H, He N, Li X, Zhou L, Zhao M, Jiang H, Zhang X. Oleanolic acid inhibits proliferation and induces apoptosis in NB4 cells by targeting PML/RARα. Oncol Lett. 2013; 6:885–890. PMID: 24137431.
Article12. Wang X, Bai H, Zhang X, Liu J, Cao P, Liao N, Zhang W, Wang Z, Hai C. Inhibitory effect of oleanolic acid on hepatocellular carcinoma via ERK-p53-mediated cell cycle arrest and mitochondrial-dependent apoptosis. Carcinogenesis. 2013; 34:1323–1330. PMID: 23404993.
Article13. Wei J, Liu M, Liu H, Wang H, Wang F, Zhang Y, Han L, Lin X. Oleanolic acid arrests cell cycle and induces apoptosis via ROS-mediated mitochondrial depolarization and lysosomal membrane permeabilization in human pancreatic cancer cells. J Appl Toxicol. 2013; 33:756–765. PMID: 22678527.
Article14. Huang J, Klionsky DJ. Autophagy and human disease. Cell Cycle. 2007; 6:1837–1849. PMID: 17671424.
Article15. Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nuñez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. Nomenclature Committee on Cell Death 2009. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009; 16:3–11. PMID: 18846107.
Article16. Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000; 290:1717–1721. PMID: 11099404.
Article17. Kondo Y, Kondo S. Autophagy and cancer therapy. Autophagy. 2006; 2:85–90. PMID: 16874083.18. Levy JM, Thorburn A. Targeting autophagy during cancer therapy to improve clinical outcomes. Pharmacol Ther. 2011; 131:130–141. PMID: 21440002.
Article19. Biederbick A, Kern HF, Elsässer HP. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur J Cell Biol. 1995; 66:3–14. PMID: 7750517.20. Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clavé C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Dröge W, Dron M, Dunn WA Jr, Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fésüs L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, González-Estévez C, Gorski S, Gottlieb RA, Häussinger D, He YW, Heidenreich K, Hill JA, Høyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jäättelä M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, Komatsu M, Kominami E, Kondo S, Kovács AL, Kroemer G, Kuan CY, Kumar R, Kundu M, Landry J, Laporte M, Le W, Lei HY, Lenardo MJ, Levine B, Lieberman A, Lim KL, Lin FC, Liou W, Liu LF, Lopez-Berestein G, López-Otín C, Lu B, Macleod KF, Malorni W, Martinet W, Matsuoka K, Mautner J, Meijer AJ, Meléndez A, Michels P, Miotto G, Mistiaen WP, Mizushima N, Mograbi B, Monastyrska I, Moore MN, Moreira PI, Moriyasu Y, Motyl T, Münz C, Murphy LO, Naqvi NI, Neufeld TP, Nishino I, Nixon RA, Noda T, Nürnberg B, Ogawa M, Oleinick NL, Olsen LJ, Ozpolat B, Paglin S, Palmer GE, Papassideri I, Parkes M, Perlmutter DH, Perry G, Piacentini M, Pinkas-Kramarski R, Prescott M, Proikas-Cezanne T, Raben N, Rami A, Reggiori F, Rohrer B, Rubinsztein DC, Ryan KM, Sadoshima J, Sakagami H, Sakai Y, Sandri M, Sasakawa C, Sass M, Schneider C, Seglen PO, Seleverstov O, Settleman J, Shacka JJ, Shapiro IM, Sibirny A, Silva-Zacarin EC, Simon HU, Simone C, Simonsen A, Smith MA, Spanel-Borowski K, Srinivas V, Steeves M, Stenmark H, Stromhaug PE, Subauste CS, Sugimoto S, Sulzer D, Suzuki T, Swanson MS, Tabas I, Takeshita F, Talbot NJ, Tallóczy Z, Tanaka K, Tanaka K, Tanida I, Taylor GS, Taylor JP, Terman A, Tettamanti G, Thompson CB, Thumm M, Tolkovsky AM, Tooze SA, Truant R, Tumanovska LV, Uchiyama Y, Ueno T, Uzcátegui NL, van der Klei I, Vaquero EC, Vellai T, Vogel MW, Wang HG, Webster P, Wiley JW, Xi Z, Xiao G, Yahalom J, Yang JM, Yap G, Yin XM, Yoshimori T, Yu L, Yue Z, Yuzaki M, Zabirnyk O, Zheng X, Zhu X, Deter RL. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008; 4:151–175. PMID: 18188003.
Article21. Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009; 137:1001–1004. PMID: 19524504.
Article22. Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008; 15:171–182. PMID: 17917680.
Article23. Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007; 17:422–427. PMID: 17804237.
Article24. Ueda S, Masutani H, Nakamura H, Tanaka T, Ueno M, Yodoi J. Redox control of cell death. Antioxid Redox Signal. 2002; 4:405–414. PMID: 12215208.
Article25. Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, Nelson DA, Jin S, White E. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006; 10:51–64. PMID: 16843265.
Article26. Zhu K, Dunner K Jr, McConkey DJ. Proteasome inhibitors activate autophagy as a cytoprotective response in human prostate cancer cells. Oncogene. 2010; 29:451–462. PMID: 19881538.
Article27. Wu Z, Chang PC, Yang JC, Chu CY, Wang LY, Chen NT, Ma AH, Desai SJ, Lo SH, Evans CP, Lam KS, Kung HJ. Autophagy blockade sensitizes prostate cancer cells towards src family kinase inhibitors. Genes Cancer. 2010; 1:40–49. PMID: 20811583.
Article28. Kim EH, Sohn S, Kwon HJ, Kim SU, Kim MJ, Lee SJ, Choi KS. Sodium selenite induces superoxide-mediated mitochondrial damage and subsequent autophagic cell death in malignant glioma cells. Cancer Res. 2007; 67:6314–6324. PMID: 17616690.
Article29. Goussetis DJ, Gounaris E, Platanias LC. BCR-ABL1-induced leukemogenesis and autophagic targeting by arsenic trioxide. Autophagy. 2013; 9:93–94. PMID: 23051894.
Article30. Lou Z, Ren T, Peng X, Sun Y, Jiao G, Lu Q, Zhang S, Lu X, Guo W. Bortezomib induces apoptosis and autophagy in osteosarcoma cells through mitogen-activated protein kinase pathway in vitro. J Int Med Res. 2013; 41:1505–1519. PMID: 23975859.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Induction of Hepatocellular Carcinoma Cell Cycle Arrest and Apoptosis by Dendropanax morbifera Leveille Leaf Extract via the PI3K/AKT/mTOR Pathway
- Myricetin Inhibits Angiogenesis by Inducing Apoptosis and Suppressing PI3K/Akt/mTOR Signaling in Endothelial Cells
- The Role of PI3K/AKT Pathway and NADPH Oxidase 4 in Host ROS Manipulation by Toxoplasma gondii
- Anti-proliferative Effect of 15,16-Dihydrotanshinone I Through Cell Cycle Arrest and the Regulation of AMP-activated Protein Kinase/Akt/mTOR and Mitogen-activated Protein Kinase Signaling Pathway in Human Hepatocellular Carcinoma Cells
- Chrysophanic Acid Induces Necrosis but not Necroptosis in Human Renal Cell Carcinoma Caki-2 Cells