J Dent Anesth Pain Med.
2016 Sep;16(3):175-184. 10.17245/jdapm.2016.16.3.175.
Remifentanil induces autophagy and prevents hydrogen peroxide-induced apoptosis in Cos-7 cells
- Affiliations
-
- 1Department of Dental Anesthesia and Pain Medicine, Pusan National University Dental Hospital, Yangsan, Korea. wjdental@hanmail.net
- 2Department of Anesthesia and Pain Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea.
- KMID: 2354652
- DOI: http://doi.org/10.17245/jdapm.2016.16.3.175
Abstract
- BACKGROUND
This study investigated the effect of remifentanil pretreatment on Cos-7 cells exposed to oxidative stress, and the influence of remifentanil on intracellular autophagy and apoptotic cell death.
METHODS
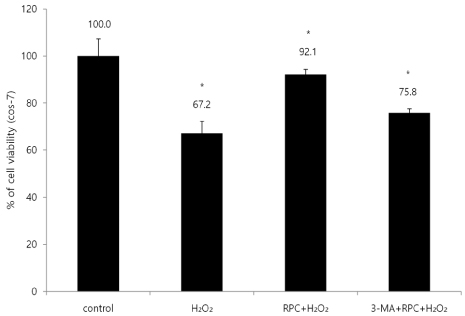
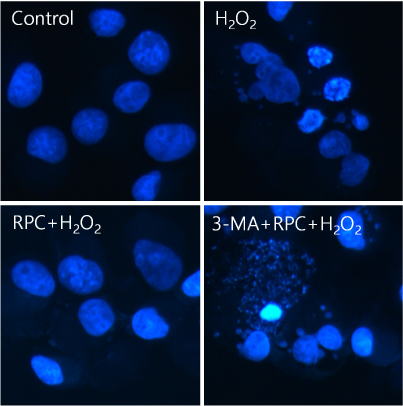
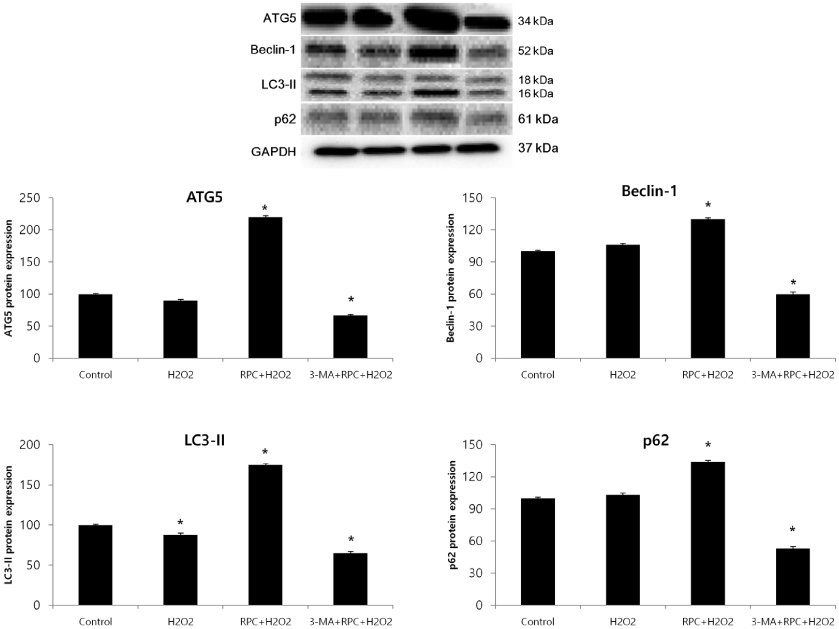
Cells were divided into 4 groups: (1) Control: non-pretreated cells were incubated in normoxia (5% COâ‚‚, 21% Oâ‚‚, and 74% Nâ‚‚). (2) Hâ‚‚Oâ‚‚: non-pretreated cells were exposed to Hâ‚‚Oâ‚‚ for 24 h. (3) RPC+Hâ‚‚Oâ‚‚: cells pretreated with remifentanil were exposed to Hâ‚‚Oâ‚‚ for 24 h. (4) 3-MA+RPC+Hâ‚‚Oâ‚‚: cells pretreated with 3-Methyladenine (3-MA) and remifentanil were exposed to Hâ‚‚Oâ‚‚ for 24 h. We determined the cell viability of each group using an MTT assay. Hoechst staining and FACS analysis of Cos-7 cells were performed to observe the effect of remifentanil on apoptosis. Autophagy activation was determined by fluorescence microscopy, MDC staining, and AO staining. The expression of autophagy-related proteins was observed using western blotting.
RESULTS
Remifentanil pretreatment increased the viability of Cos-7 cells exposed to oxidative stress. Hoechst staining and FACS analysis revealed that oxidative stress-dependent apoptosis was suppressed by the pretreatment. Additionally, fluorescence microscopy showed that remifentanil pretreatment led to autophagy-induction in Cos-7 cells, and the expression of autophagy-related proteins was increased in the RPC+Hâ‚‚Oâ‚‚ group.
CONCLUSIONS
The study showed that remifentanil pretreatment stimulated autophagy and increased viability in an oxidative stress model of Cos-7 cells. Therefore, we suggest that apoptosis was activated upon oxidative stress, and remifentanil preconditioning increased the survival rate of the cells by activating autophagy.
Keyword
MeSH Terms
Figure
Reference
-
1. Elahi MM, Kong YX, Matata BM. Oxidative stress as a mediator of cardiovascular disease. Oxid Med Cell Longev. 2009; 2:259–269.
Article2. Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002; 82:47–95.
Article3. Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006; 141:312–322.
Article4. Valko M, Rhodes C, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006; 160:1–40.
Article5. Zhao Y, Zhao B. Oxidative stress and the pathogenesis of alzheimer's disease. Oxid Med Cell Longev. 2013; 2013:316523.
Article6. Singh DK, Winocour P, Farrington K. Oxidative stress in early diabetic nephropathy: fueling the fire. Nat Rev Endocrinol. 2011; 7:176–184.
Article7. Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005; 115:500–508.
Article8. Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, et al. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007; 9:49–89.9. Mizushima N. The pleiotropic role of autophagy: From protein metabolism to bactericide. Cell Death Differ. 2005; 12:1535–1541.10. Guan X, Qian Y, Shen Y, Zhang L, Du Y, Dai H, et al. Autophagy protects renal tubular cells against ischemia/reperfusion injury in a time-dependent manner. Cell Physiol Biochem. 2015; 36:285–298.
Article11. Kim YH, Kang JM, Kim IR, Lee BY, Yoon JY, Kim CH, et al. Protective effect of propofol against hypoxia-reoxygenation injury in HaCaT human keratinocytes. Int J Oral Biol. 2014; 39:97–105.
Article12. Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: Physiology and pathology. Trends Biochem Sci. 2011; 36:30–38.
Article13. Kwon JY, Park BS, Kim YH, Kim YD, Kim CH, Yoon JY, et al. Remifentanil protects human keratinocytes against hypoxia-reoxygenation injury through activation of autophagy. PloS One. 2015; 10:e0116982.
Article14. Scott LJ, Perry CM. Spotlight on remifentanil for general anaesthesia. CNS drugs. 2005; 19:1069–1074.
Article15. Komatsu R, Turan A, Orhan-Sungur M, McGuire J, Radke O, Apfel C. Remifentanil for general anaesthesia: A systematic review. Anaesthesia. 2007; 62:1266–1280.
Article16. Marsh D, Hodkinson B. Remifentanil in paediatric anaesthetic practice. Anaesthesia. 2009; 64:301–308.
Article17. Cho SS, Rudloff I, Berger PJ, Irwin MG, Nold MF, Cheng W, et al. Remifentanil ameliorates intestinal ischemia-reperfusion injury. BMC Gastroenterol. 2013; 13:69.
Article18. Zhang Y, Irwin MG, Wong TM, Chen M, Cao CM. Remifentanil preconditioning confers cardioprotection via cardiac kappa- and delta-opioid receptors. Anesthesiology. 2005; 102:371–378.
Article19. Kim H, Cho J, Hong S, Kim S, Shim J, Kwak Y. Remifentanil protects myocardium through activation of anti-apoptotic pathways of survival in ischemia-reperfused rat heart. Physiol Res. 2010; 59:347–356.
Article20. Zhang TZ, Zhou J, Jin Q, Sun YJ, Diao YG, Zhang YN, et al. Protective effects of remifentanil preconditioning on cerebral injury during pump-assisted coronary artery bypass graft. Genet Mol Res. 2014; 13:7658–7665.
Article21. Chang C, Chen P, Lu S, Hsieh M, Lin C, Lee H, et al. Propofol-enhanced autophagy increases motility and angiogenic capacity of cultured human umbilical vascular endothelial cells. Life Sci. 2015; 142:49–59.
Article22. Anding AL, Baehrecke EH. Chapter three-autophagy in cell life and cell death. Curr Top Dev Biol. 2015; 114:67–91.
Article23. Baehrecke EH. Autophagy: Dual roles in life and death? Nat Rev Mol Cell Biol. 2005; 6:505–510.
Article24. Fitzwalter BE, Thorburn A. Recent insights into cell death and autophagy. FEBS J. 2015; 282:4279–4288.
Article25. Levine B, Yuan J. Autophagy in cell death: An innocent convict? J Clin Invest. 2005; 115:2679–2688.
Article26. Huang C, Lee C, Lin H, Chen M, Lin C, Chang J. Autophagy-regulated ROS from xanthine oxidase acts as an early effector for triggering late mitochondria-dependent apoptosis in cathepsin S-targeted tumor cells. PloS one. 2015; 10:e0128045.
Article27. Elahi M, Matata B. Free radicals in blood: Evolving concepts in the mechanism of ischemic heart disease. Arch Biochem Biophys. 2006; 450:78–88.
Article28. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000; 408:239–247.
Article29. Yue Z, Friedman L, Komatsu M, Tanaka K. The cellular pathways of neuronal autophagy and their implication in neurodegenerative diseases. Biochim Biophys Acta. 2009; 1793:1496–1507.
Article30. Yan L, Sadoshima J, Vatner DE, Vatner SF. Autophagy: A novel protective mechanism in chronic ischemia. Cell Cycle. 2006; 5:1175–1177.
Article31. Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: Physiology and pathology. Trends Biochem Sci. 2011; 36:30–38.
Article32. Zhao G, Shen X, Nan H, Yan L, Zhao H, Yu J, et al. Remifentanil protects liver against ischemia/reperfusion injury through activation of anti-apoptotic pathways. J Surg Res. 2013; 183:827–834.
Article33. Peart JN, Gross ER, Gross GJ. Opioid-induced preconditioning: Recent advances and future perspectives. Vascul Pharmacol. 2005; 42:211–218.
Article34. Headrick JP, See Hoe LE, Du Toit EF, Peart JN. Opioid receptors and cardioprotection–'opioidergic conditioning' of the heart. Br J Pharmacol. 2015; 172:2026–2050.
Article35. Costa-Malaquias A, Almeida MB, Monteiro JRS, de Matos Macchi B, do Nascimento JLM, Crespo-Lopez ME. Morphine protects against methylmercury intoxication: A role for opioid receptors in oxidative stress? PloS One. 2014; 9:e110815.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Propofol protects against oxidative-stress-induced COS-7 cell apoptosis by inducing autophagy
- Effects of Remifentanil Preconditioning Attenuating Oxidative Stress in Human Dermal Fibroblast
- Activation of Caspase-3 in Hydrogen Peroxide-Induced Apoptosis of Human Leukemia HL 60 Cells
- Induction of Apoptosis in Synovial Cells from Patients with Rheumatoid Arthritis
- Propofol protects human keratinocytes from oxidative stress via autophagy expression