Infect Chemother.
2008 Aug;40(4):212-217. 10.3947/ic.2008.40.4.212.
In Vitro Antibiotic Susceptibility of Orientia tsutsugamushi strain Boryong Measured by Flow Cytometry
- Affiliations
-
- 1Department of Internal Medicine, DaeSung General Hospital, Buchon, Korea.
- 2Clinical Research Center, Inha University College of Medicine, Inchon, Korea.
- 3Department of Internal Medicine, Inha University College of Medicine, Inchon, Korea.
- 4Department of Microbiology, Inha University College of Medicine, Inchon, Korea.
- KMID: 2170236
- DOI: http://doi.org/10.3947/ic.2008.40.4.212
Abstract
-
BACKGROUND: Scrub typhus, an infectious disease caused by Orientia tsutsugamushi, is endemic in Korea. With the introduction of tetracycline and chloramphenicol in clinical practice, the mortality due to scrub typhus has markedly decreased. In 1995, scrub typhus poorly responsive to doxycycline was reported in Thailand; the need for safe antibiotics for the treatment of scrub typhus acquired during pregnancy or for children is emerging; also, broader spectrum antibiotics having anti-Orientia activity may be preferred for empirical therapy of enteric fever syndrome and for complicated scrub typhus. The anti-Orientia activities of various antibiotics, including recently licensed antibiotics, were investigated by flow cytometry.
MATERIALS AND METHODS
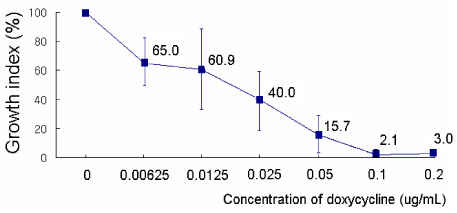
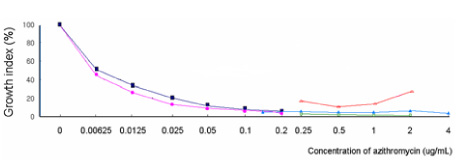
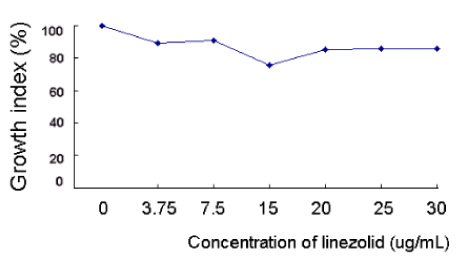
O. tsutsugamushi strain Boryong was inoculated into the ECV304 cell line. The infected cells were stained with FS15, a monoclonal antibody reacting against a linear epitope on 56-kDa major outer membrane protein of O. tsutsugamushi. Then the antimicrobial susceptibilities were measured by flow cytometry and expressed as a growth index (total mass of Orientia). A concentration at which no further decrease in growth index occurred was defined as the minimal inhibitory concentration (MIC). Microbial susceptibilities to the following antibiotics were measured: quinupristin-dalfopristin (Synercid), levofloxacin, ciprofloxacin, moxifloxacin, metronidazole, linezolid, clindamycin, chloramphenicol, doxycycline, azithromycin, and rifampin. RESULTS: Considering the usual serum concentrations of rifampin (MIC=0.025-0.05 microg/mL), azithromycin (MIC=0.05-0.5 microg/mL) and doxycycline (MIC=0.05-0.1 microg/mL), these antibiotics exhibited very low MICs. Synercid (MIC=0.2-1.0 microg/mL), clindamycin (MIC=1.0 microg/mL) and chloramphenicol (MIC=1-2 microg/mL) exhibited moderately low MICs; moxifloxacin (MIC=8 microg/mL), ciprofloxacin (MIC=25.6 microg/mL or more) and levofloxacin (MIC=30 microg/mL) exhibited relatively high MICs; and cefotaxime (MIC>50 microg/mL), metronidazole (MIC>30 microg/mL) and linezolid (>30 microg/mL) exhibited high MICs.
CONCLUSIONS
Among the new antibiotics, none was superior to doxycycline, azithromycin or rifampin with respect to anti-Orientia activity. Synercid, clindamycin, and moxifloxacin may show moderate therapeutic efficacies in human.
MeSH Terms
-
Acetamides
Anti-Bacterial Agents
Aza Compounds
Azithromycin
Cefotaxime
Cell Line
Child
Chloramphenicol
Ciprofloxacin
Clindamycin
Communicable Diseases
Doxycycline
Flow Cytometry
Humans
Korea
Linezolid
Membrane Proteins
Metronidazole
Ofloxacin
Orientia tsutsugamushi
Oxazolidinones
Pregnancy
Quinolines
Rifampin
Scrub Typhus
Sprains and Strains
Tetracycline
Typhoid Fever
Virginiamycin
Acetamides
Anti-Bacterial Agents
Aza Compounds
Azithromycin
Cefotaxime
Chloramphenicol
Ciprofloxacin
Clindamycin
Doxycycline
Membrane Proteins
Metronidazole
Ofloxacin
Oxazolidinones
Quinolines
Rifampin
Tetracycline
Virginiamycin
Figure
Cited by 3 articles
-
Doxycycline Resistance in Orientia tsutsugamushi Isolated from Korean Patients
Eun Sil Kim, Mee Kyung Kim, Hye Myung Lee, Se-Hee Kil, Moon-Hyun Chung, Jin-Soo Lee, Jae-Seung Kang
Infect Chemother. 2008;40(5):259-265. doi: 10.3947/ic.2008.40.5.259.In vitro Efficacy of Antibiotic Combinations against Orientia tsutsugamushi
Eun Sil Kim, Mee Kyung Kim, Hye Myung Lee, Moon-Hyun Chung, Jin-Soo Lee, Jae-Seung Kang
Infect Chemother. 2008;40(6):311-315. doi: 10.3947/ic.2008.40.6.311.In vitro Synergism between Chloroquine and Antibiotics against Orientia tsutsugamushi
Dongwook Son, Moon-Hyun Chung
Infect Chemother. 2014;46(3):182-188. doi: 10.3947/ic.2014.46.3.182.
Reference
-
1. Machella TE, Forrester JS. Mite or scrub typhus: a clinical and laboratory study of 64 cases. Am J Med Sci. 1945. 38:38–61.
Article2. Sayen J, Pond H, Forrester J. Scrub typhus in Assam and Burma: a clinical study of 616 cases. Medicine. 1946. 25:155–214.
Article3. Strickman D, Sheer T, Salata K, Hershey J, Dasch G, Kelly D, Kuschner R. In vitro effectiveness of azithromycin against doxycycline-resistant and -susceptible strains of Rickettsia tsutsugamushi, etiologic agent of scrub typhus. Antimicrob Agents Chemother. 1995. 39:2406–2410.
Article4. Watt G, Chouriyagune C, Ruangweerayud R, Watcharapichat P, Phulsuksombati D, Jongsakul K, Teja-Isavadharm P, Bhodhidatta D, Corcoran KD, Dasch GA, Strickman D. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet. 1996. 348:86–89.
Article5. Kim MJ, Kim MK, Kang JS. Improved antibiotic susceptibility test of Orientia tsutsugamushi by flow cytometry using monoclonal antibody. J Korean Med Sci. 2007. 22:1–6.
Article6. Chang WH, Kang JS, Lee WK, Choi MS, Lee JH. Serological classification by monoclonal antibodies of Rickettsia tsutsugamushi isolated in Korea. J Clin Microbiol. 1990. 28:685–688.
Article7. Suchland RJ, Geisler WM, Stamm WE. Methodologies and cell lines used for antimicrobial susceptibility testing of Chlamydia spp. Antimicrob Agents Chemother. 2003. 47:636–642.
Article8. Smadel JE, Jackson EB, Cruise AB. Chloromycetin in experimental rickettsial infections. J Immunol. 1949. 62:49–65.
Article9. Wong SC, Cox HR. Action of aureomycin against experimental rickettsial and viral infections. Ann NY Acad Sci. 1948. 51:290–305.
Article10. McDade JE. Determination of antibiotic susceptibility of Rickettsia by the plaque assay technique. Appl Microbiol. 1969. 18:133–135.
Article11. Raoult D, Roussellier P, Vestris G, Tamalet J. In vitro antibiotic susceptibility of Rickettsia rickettsii and Rickettsia conorii: plaque assay and microplaque colorimetric assay. J Infect Dis. 1987. 155:1059–1062.
Article12. Kelly DJ, Salata KF, Strickman D, Hershey JN. Rickettsia tsutsugamushi infection in cell culture: antibiotic susceptibility determined by flow cytometry. Am J Trop Med Hyg. 1995. 53:602–606.
Article13. Branger S, Rolain JM, Raoult D. Evaluation of antibiotic susceptibilities of Ehrlichia canis, Ehrlichia chaffeensis, and Anaplasma phagocytophilum by real-time PCR. Antimicrob Agents Chemother. 2004. 48:4822–4828.
Article14. Miyamura S, Sato N, Tamura A. In vitro susceptibility of recent clinical isolates of Rickettsia tsutsugamushi to chemotherapeutic agents. Kansenshogaku Zasshi. 1985. 59:486–488.
Article15. Miyamura S, Ohta T, Tamura A. Comparison of in vitro susceptibilities of Rickettsia prowazekii, R. rickettsii, R. sibirica and R. tsutsugamushi to antimicrobial agents. Nippon Saikingaku Zasshi. 1989. 44:717–721.
Article16. Raoult D, Drancourt M. Antimicrobial therapy of rickettsial diseases. Antimicrob Agents Chemother. 1991. 35:2457–2462.
Article17. Eaton M, Cohen MT, Shlim DR, Innes B. Ciprofloxacin treatment of typhus. JAMA. 1989. 262:772–773.
Article18. Jee HG, Chung MH, Lee SG, Kim IS, Chang WH. Transmission of scrub typhus by needlestick from a patient receiving pefloxacin. Scand J Infect Dis. 1996. 28:411–412.
Article19. Jensenius M, Montelius R, Berild D, Vene S. Scrub typhus imported to Scandinavia. Scand J Infect Dis. 2006. 38:200–202.20. Mathai E, Rolain JM, Verghese L, Mathai M, Jasper P, Verghese G, Raoult D. Case reports: scrub typhus during pregnancy in India. Trans R Soc Trop Med Hyg. 2003. 97:570–572.21. Oh SY, Chung MH, Oh SJ, Son MS, Ahn SW. An open clinical trial to compare the efficacy of ciprofloxacin, pefloxacin, and doxycycline in the treatment of scrub typhus. Korean J Infect Dis. 1995. 27:193–198.
Article22. Kim MK, Odgerel Z, Kim MJ, Chung MH, Lim BU, Kang JS. Application of monoclonal antibody, specific for intracellular Orientia tsutsugamushi, to immunofluorescent antibody test for determining antibiotic susceptibility. Microbiol Immunol. 2004. 48:655–660.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Improved Antibiotic Susceptibility Test of Orientia tsutsugamushi by Flow Cytometry Using Monoclonal Antibody
- In vitro Study on the Dose and Duration of Doxycycline Treatment against Orientia tsutsugamushi
- Analysis of antigenic characteristics of Rickettsia tsutsugamushi Boryong strain and antigenic heterogeneity of Rickettsia tsutsugamushi using monoclonal antibodies
- In vitro Efficacy of Antibiotic Combinations against Orientia tsutsugamushi
- In Vitro Bacteriostatic Effects of Rifampin on Orientia tsutsugamushi