J Periodontal Implant Sci.
2017 Oct;47(5):273-291. 10.5051/jpis.2017.47.5.273.
Static magnetic fields promote osteoblastic/cementoblastic differentiation in osteoblasts, cementoblasts, and periodontal ligament cells
- Affiliations
-
- 1Department of Oral and Maxillofacial Pathology, Institute of Oral Biology, Kyung Hee University School of Dentistry, Seoul, Korea.
- 2Department of Dental Materials, Kyung Hee University School of Dentistry, Seoul, Korea.
- 3Department of Biomaterials and Prosthodontics, Kyung Hee University Hospital at Gangdong, Kyung Hee University School of Dentistry, Seoul, Korea. hswhsh@khu.ac.kr
- KMID: 2392716
- DOI: http://doi.org/10.5051/jpis.2017.47.5.273
Abstract
- PURPOSE
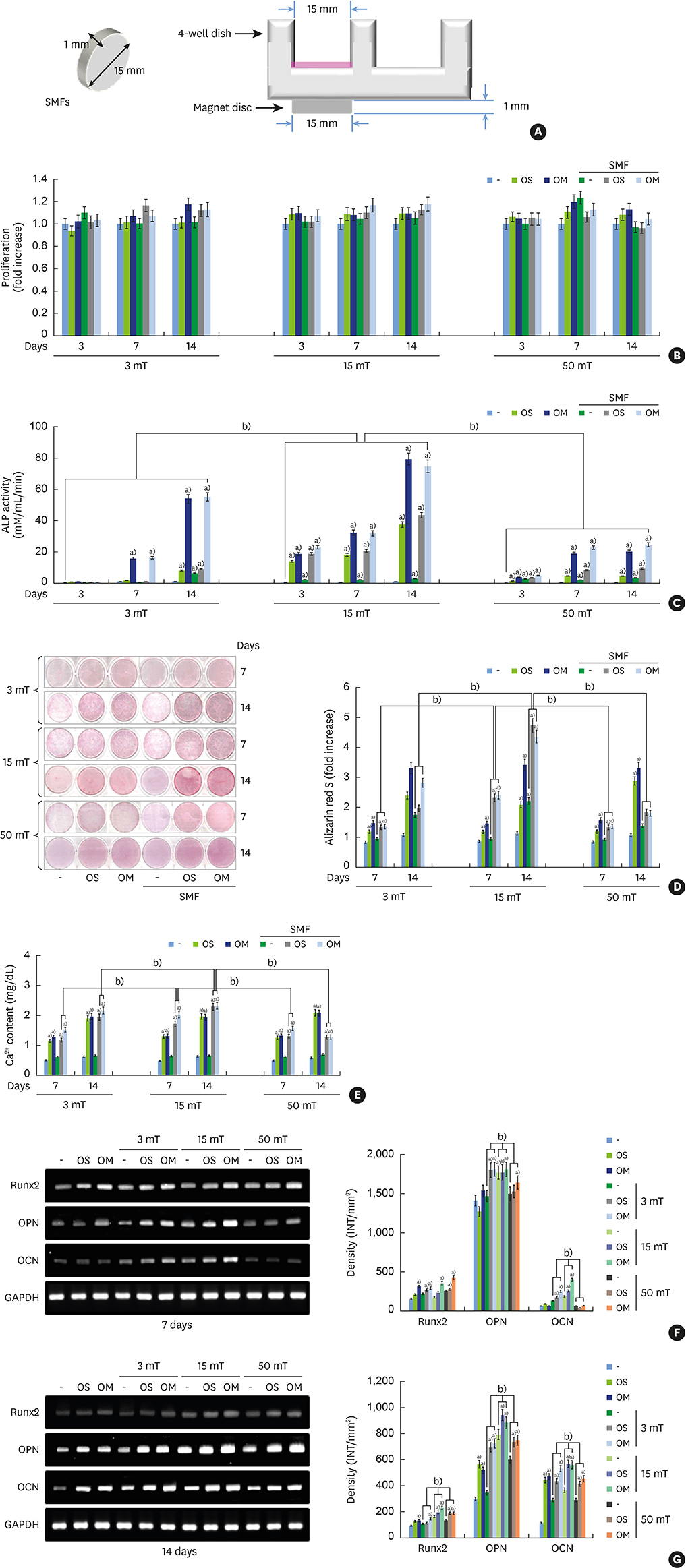
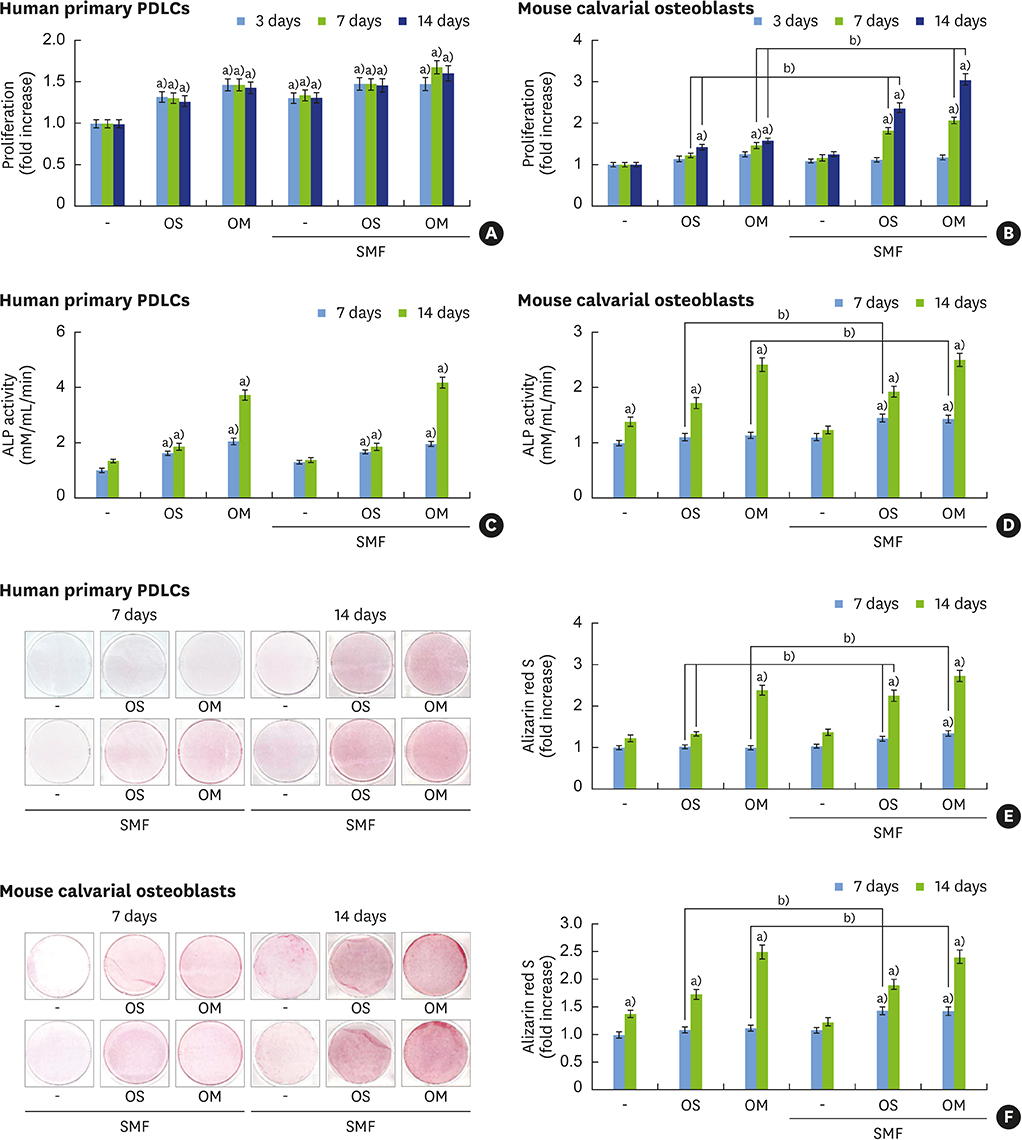
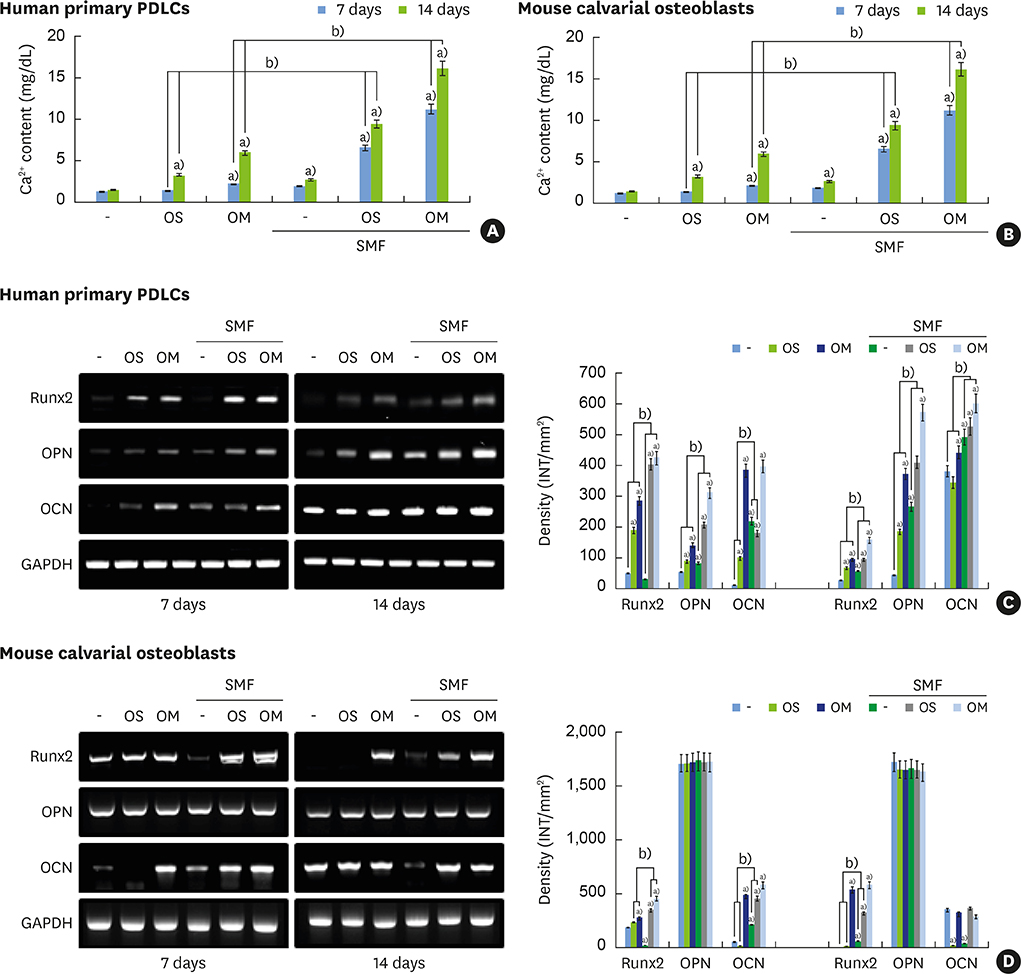
Although static magnetic fields (SMFs) have been used in dental prostheses and osseointegrated implants, their biological effects on osteoblastic and cementoblastic differentiation in cells involved in periodontal regeneration remain unknown. This study was undertaken to investigate the effects of SMFs (15 mT) on the osteoblastic and cementoblastic differentiation of human osteoblasts, periodontal ligament cells (PDLCs), and cementoblasts, and to explore the possible mechanisms underlying these effects.
METHODS
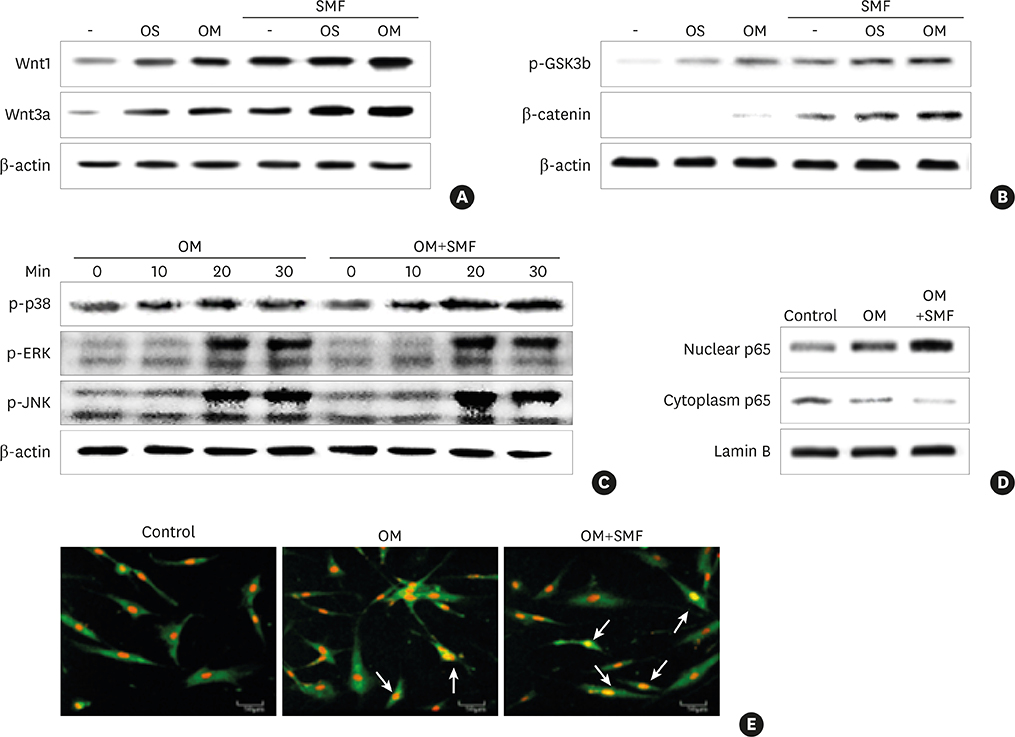
Differentiation was evaluated by measuring alkaline phosphatase (ALP) activity, mineralized nodule formation based on Alizarin red staining, calcium content, and the expression of marker mRNAs assessed by reverse transcription polymerase chain reaction (RT-PCR). Signaling pathways were analyzed by western blotting and immunocytochemistry.
RESULTS
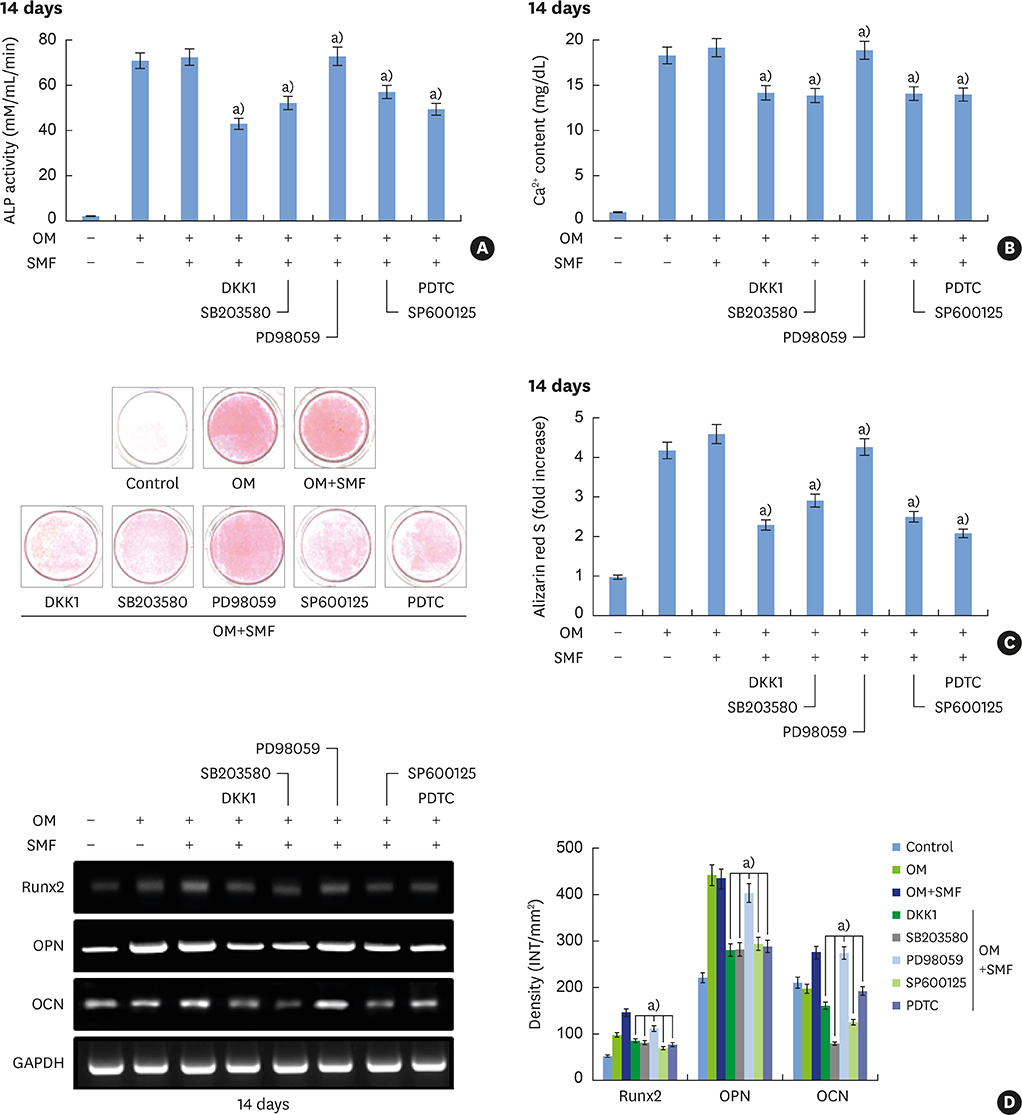
The activities of the early marker ALP and the late markers matrix mineralization and calcium content, as well as osteoblast- and cementoblast-specific gene expression in osteoblasts, PDLCs, and cementoblasts were enhanced. SMFs upregulated the expression of Wnt proteins, and increased the phosphorylation of glycogen synthase kinase-3β (GSK-3β) and total β-catenin protein expression. Furthermore, p38 and c-Jun N-terminal kinase (JNK) mitogen-activated protein kinase (MAPK), and nuclear factor-κB (NF-κB) pathways were activated.
CONCLUSIONS
SMF treatment enhanced osteoblastic and/or cementoblastic differentiation in osteoblasts, cementoblasts, and PDLCs. These findings provide a molecular basis for the beneficial osteogenic and/or cementogenic effect of SMFs, which could have potential in stimulating bone or cementum formation during bone regeneration and in patients with periodontal disease.
Keyword
MeSH Terms
-
Alkaline Phosphatase
Blotting, Western
Bone Regeneration
Calcium
Dental Cementum*
Dental Prosthesis
Gene Expression
Glycogen Synthase
Guided Tissue Regeneration, Periodontal
Humans
Immunohistochemistry
JNK Mitogen-Activated Protein Kinases
Magnetic Fields*
Miners
Osteoblasts*
Periodontal Diseases
Periodontal Ligament*
Phosphorylation
Polymerase Chain Reaction
Protein Kinases
Regeneration
Relative Biological Effectiveness
Reverse Transcription
RNA, Messenger
Signal Transduction
Wnt Proteins
Alkaline Phosphatase
Calcium
Glycogen Synthase
JNK Mitogen-Activated Protein Kinases
Protein Kinases
RNA, Messenger
Wnt Proteins
Figure
Reference
-
1. Shimono M, Ishikawa T, Ishikawa H, Matsuzaki H, Hashimoto S, Muramatsu T, et al. Regulatory mechanisms of periodontal regeneration. Microsc Res Tech. 2003; 60:491–502.
Article2. Cook JJ, Summers NJ, Cook EA. Healing in the new millennium: bone stimulators: an overview of where we’ve been and where we may be heading. Clin Podiatr Med Surg. 2015; 32:45–59.3. Funk RH, Monsees T, Ozkucur N. Electromagnetic effects - from cell biology to medicine. Prog Histochem Cytochem. 2009; 43:177–264.
Article4. Gaetani R, Ledda M, Barile L, Chimenti I, De Carlo F, Forte E, et al. Differentiation of human adult cardiac stem cells exposed to extremely low-frequency electromagnetic fields. Cardiovasc Res. 2009; 82:411–420.
Article5. Sakata M, Yamamoto Y, Imamura N, Nakata S, Nakasima A. The effects of a static magnetic field on orthodontic tooth movement. J Orthod. 2008; 35:249–254.
Article6. Zhang J, Ding C, Ren L, Zhou Y, Shang P. The effects of static magnetic fields on bone. Prog Biophys Mol Biol. 2014; 114:146–152.
Article7. Markov MS. Magnetic field therapy: a review. Electromagn Biol Med. 2007; 26:1–23.
Article8. Yang TC, Maeda Y, Gonda T, Wada M. Magnetic attachment for implant overdentures: influence of contact relationship with the denture base on stability and bending strain. Int J Prosthodont. 2013; 26:563–565.
Article9. Aksu AE, Dursun E, Calis M, Ersu B, Safak T, Tözüm TF. Intraoral use of extraoral implants for oral rehabilitation of a pediatric patient after resection of ewing sarcoma of the mandible and reconstruction with iliac osteocutaneous free flap. J Craniofac Surg. 2014; 25:930–933.
Article10. Siadat H, Bassir SH, Alikhasi M, Shayesteh YS, Khojasteh A, Monzavi A. Effect of static magnetic fields on the osseointegration of immediately placed implants: a randomized controlled clinical trial. Implant Dent. 2012; 21:491–495.
Article11. Leesungbok R, Ahn SJ, Lee SW, Park GH, Kang JS, Choi JJ. The effects of a static magnetic field on bone formation around a sandblasted, large-grit, acid-etched–treated titanium implant. J Oral Implantol. 2013; 39:248–255.
Article12. Yamamoto Y, Ohsaki Y, Goto T, Nakasima A, Iijima T. Effects of static magnetic fields on bone formation in rat osteoblast cultures. J Dent Res. 2003; 82:962–966.
Article13. Denaro V, Papapietro N, Sgambato A, Barnaba SA, Ruzzini L, Paola BD, et al. Periprosthetic electrochemical corrosion of titanium and titanium-based alloys as a cause of spinal fusion failure. Spine. 2008; 33:8–13.
Article14. Denaro V, Cittadini A, Barnaba SA, Ruzzini L, Denaro L, Rettino A, et al. Static electromagnetic fields generated by corrosion currents inhibit human osteoblast differentiation. Spine. 2008; 33:955–959.
Article15. Chiu KH, Ou KL, Lee SY, Lin CT, Chang WJ, Chen CC, et al. Static magnetic fields promote osteoblast-like cells differentiation via increasing the membrane rigidity. Ann Biomed Eng. 2007; 35:1932–1939.
Article16. Hsu SH, Chang JC. The static magnetic field accelerates the osteogenic differentiation and mineralization of dental pulp cells. Cytotechnology. 2010; 62:143–155.
Article17. Kim EC, Leesungbok R, Lee SW, Lee HW, Park SH, Mah SJ, et al. Effects of moderate intensity static magnetic fields on human bone marrow-derived mesenchymal stem cells. Bioelectromagnetics. 2015; 36:267–276.
Article18. Bartold PM, Narayanan AS. Molecular and cell biology of healthy and diseased periodontal tissues. Periodontol 2000. 2006; 40:29–49.
Article19. Miyakoshi J. Effects of static magnetic fields at the cellular level. Prog Biophys Mol Biol. 2005; 87:213–223.
Article20. Wang Y, Qin QH. A theoretical study of bone remodelling under PEMF at cellular level. Comput Methods Biomech Biomed Engin. 2012; 15:885–897.
Article21. Chung JH, Kim YS, Noh K, Lee YM, Chang SW, Kim EC. Deferoxamine promotes osteoblastic differentiation in human periodontal ligament cells via the nuclear factor erythroid 2-related factor-mediated antioxidant signaling pathway. J Periodontal Res. 2014; 49:563–573.
Article22. Kitagawa M, Tahara H, Kitagawa S, Oka H, Kudo Y, Sato S, et al. Characterization of established cementoblast-like cell lines from human cementum-lining cells in vitro and in vivo . Bone. 2006; 39:1035–1042.
Article23. Pi SH, Lee SK, Hwang YS, Choi MG, Lee SK, Kim EC. Differential expression of periodontal ligament-specific markers and osteogenic differentiation in human papilloma virus 16-immortalized human gingival fibroblasts and periodontal ligament cells. J Periodontal Res. 2007; 42:104–113.
Article24. Beertsen W, McCulloch CA, Sodek J. The periodontal ligament: a unique, multifunctional connective tissue. Periodontol 2000. 1997; 13:20–40.
Article25. Chen FM, Jin Y. Periodontal tissue engineering and regeneration: current approaches and expanding opportunities. Tissue Eng Part B Rev. 2010; 16:219–255.
Article26. Kim MB, Song Y, Hwang JK. Kirenol stimulates osteoblast differentiation through activation of the BMP and Wnt/β-catenin signaling pathways in MC3T3-E1 cells. Fitoterapia. 2014; 98:59–65.
Article27. Nöth U, Tuli R, Seghatoleslami R, Howard M, Shah A, Hall DJ, et al. Activation of p38 and Smads mediates BMP-2 effects on human trabecular bone-derived osteoblasts. Exp Cell Res. 2003; 291:201–211.
Article28. Franceschi RT, Xiao G. Regulation of the osteoblast-specific transcription factor, Runx2: responsiveness to multiple signal transduction pathways. J Cell Biochem. 2003; 88:446–454.
Article29. Subramaniam M, Jalal SM, Rickard DJ, Harris SA, Bolander ME, Spelsberg TC. Further characterization of human fetal osteoblastic hFOB 1.19 and hFOB/ER alpha cells: bone formation in vivo and karyotype analysis using multicolor fluorescent in situ hybridization. J Cell Biochem. 2002; 87:9–15.
Article30. Hayami T, Zhang Q, Kapila Y, Kapila S. Dexamethasone’s enhancement of osteoblastic markers in human periodontal ligament cells is associated with inhibition of collagenase expression. Bone. 2007; 40:93–104.
Article31. Bodine PV, Komm BS. Wnt signaling and osteoblastogenesis. Rev Endocr Metab Disord. 2006; 7:33–39.
Article32. Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005; 434:843–850.
Article33. Zhou J, He H, Yang L, Chen S, Guo H, Xia L, et al. Effects of pulsed electromagnetic fields on bone mass and Wnt/β-catenin signaling pathway in ovariectomized rats. Arch Med Res. 2012; 43:274–282.
Article34. Du L, Fan H, Miao H, Zhao G, Hou Y. Extremely low frequency magnetic fields inhibit adipogenesis of human mesenchymal stem cells. Bioelectromagnetics. 2014; 35:519–530.
Article35. Wolf-Goldberg T, Barbul A, Ben-Dov N, Korenstein R. Low electric fields induce ligand-independent activation of EGF receptor and ERK via electrochemical elevation of H(+) and ROS concentrations. Biochim Biophys Acta. 2013; 1833:1396–1408.
Article36. Ma J, Zhang Z, Su Y, Kang L, Geng D, Wang Y, et al. Magnetic stimulation modulates structural synaptic plasticity and regulates BDNF-TrkB signal pathway in cultured hippocampal neurons. Neurochem Int. 2013; 62:84–91.
Article37. Sun W, Yu Y, Chiang H, Fu Y, Lu D. Exposure to power-frequency magnetic fields can induce activation of P38 mitogen-activated protein kinase. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2002; 20:252–255.38. Soda A, Ikehara T, Kinouchi Y, Yoshizaki K. Effect of exposure to an extremely low frequency-electromagnetic field on the cellular collagen with respect to signaling pathways in osteoblast-like cells. J Med Invest. 2008; 55:267–278.
Article39. Vincenzi F, Targa M, Corciulo C, Gessi S, Merighi S, Setti S, et al. Pulsed electromagnetic fields increased the anti-inflammatory effect of A2A and A3 adenosine receptors in human T/C-28a2 chondrocytes and hFOB 1.19 osteoblasts. PLoS One. 2013; 8:e65561.40. Sakurai T, Terashima S, Miyakoshi J. Enhanced secretion of prostaglandin E2 from osteoblasts by exposure to a strong static magnetic field. Bioelectromagnetics. 2008; 29:277–283.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunolocalization of Runx2 and Osterix in the Developing Periodontal Tissues of the Mouse
- Effect of Extract of Seeds of Carthamus tinctorius L. on Mineralization in Periodontal Ligament Cells and Osteoblastic Cells
- The effect of dexamethasone on the gene expression of the bone matrix protein in the periodontal ligament cells
- Effect of 17beta-Estradiol and 1,25-Dihydroxyvitamin D3 on Interleukin-6 Production of Periodontal Ligament Cells
- The effect of rhBMP-2 on the osteoblastic differentiation of human periodontal ligament cells and gingival fibroblasts in vitro