Cancer Res Treat.
2017 Jul;49(3):778-789. 10.4143/crt.2015.485.
Effects and Mechanisms of Metformin on the Proliferation of Esophageal Cancer Cells In Vitro and In Vivo
- Affiliations
-
- 1Department of Biochemistry, North of Sichuan Medical University, Nanchong, China. tangjiancai1980@163.com
- 2School of Basic Medical Sciences, North of Sichuan Medical University, Nanchong, China.
- 3Pathogenic Biology and Immunology Experiment Teaching Center, North of Sichuan Medical University, Nanchong, China.
- KMID: 2388323
- DOI: http://doi.org/10.4143/crt.2015.485
Abstract
- PURPOSE
The purpose of this study was to observe the effects of metformin on human esophageal cancer cell and to investigate its possible mechanisms.
MATERIALS AND METHODS
Cell viability was detected by using a Cell Counting Kit-8, while cell cycle and apoptosis were assessed by flow cytometry and western blot was used to measure the expression of the related proteins. RNAi was used to knockout pyruvate kinase muscle isozyme 2 (PKM2). An Eca109 tumor model was established to evaluate the antitumor effect in vivo. Immunohistochemistry was determined based on the expression of PKM2 and Bim in tumor tissues. Tunnel was used to assess tumor cell apoptosis.
RESULTS
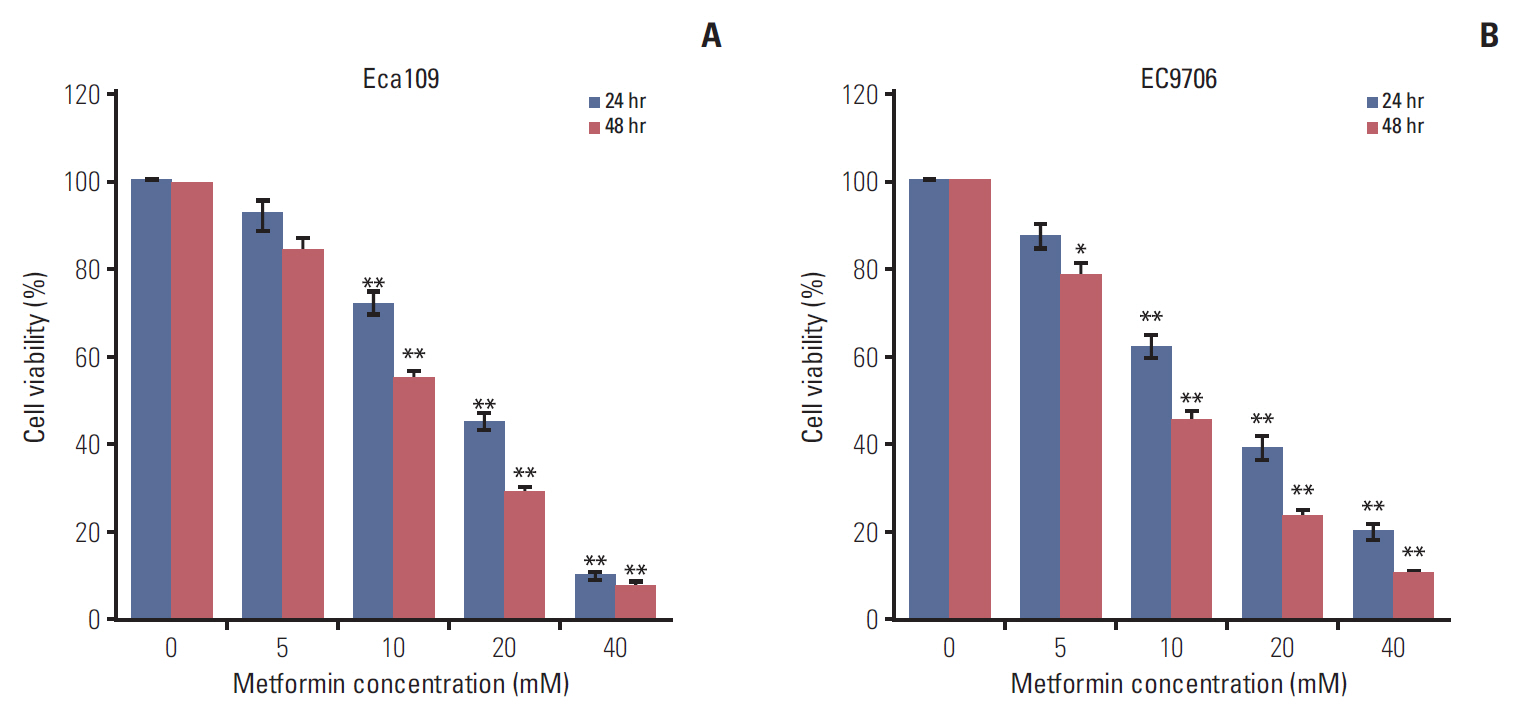
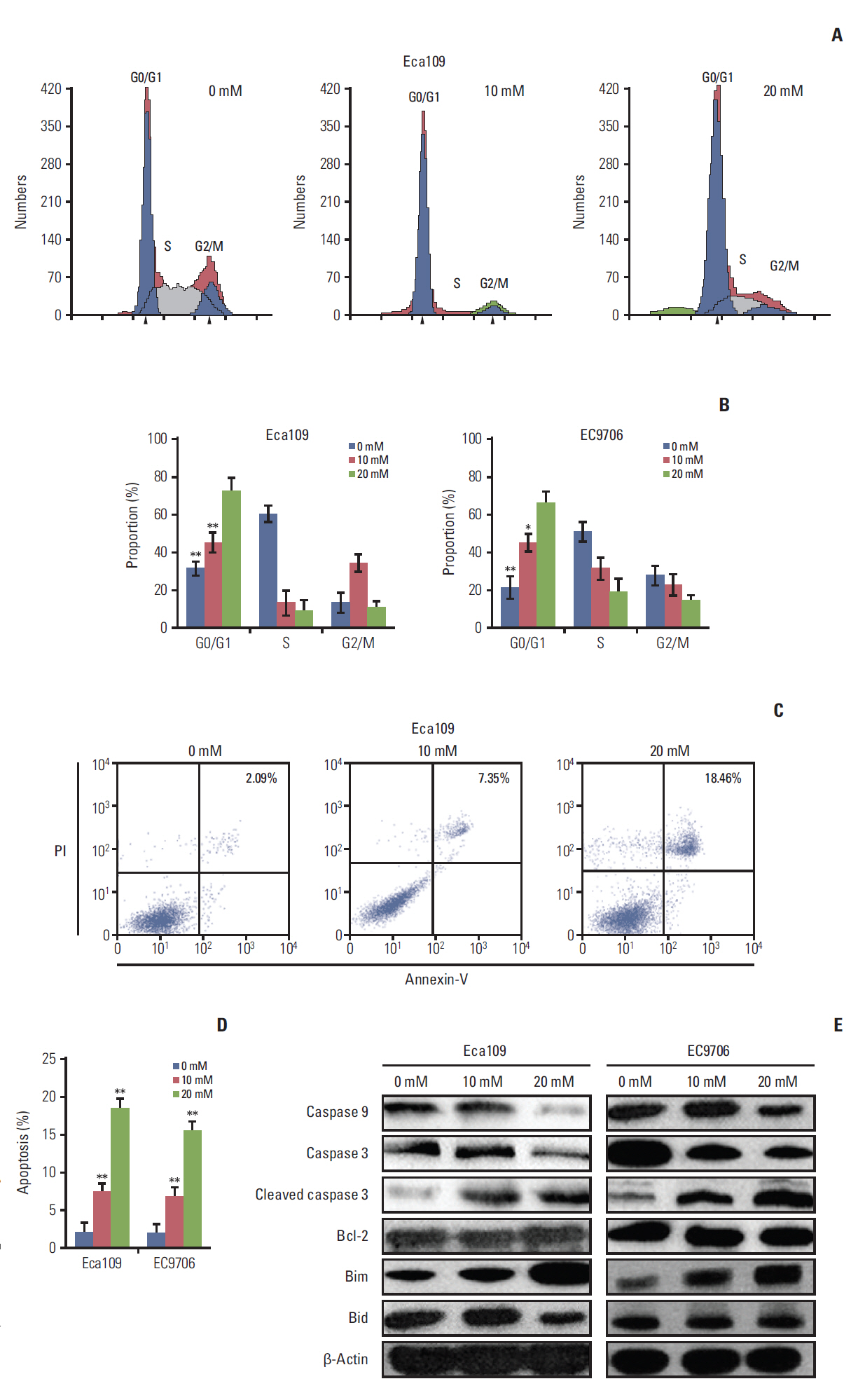
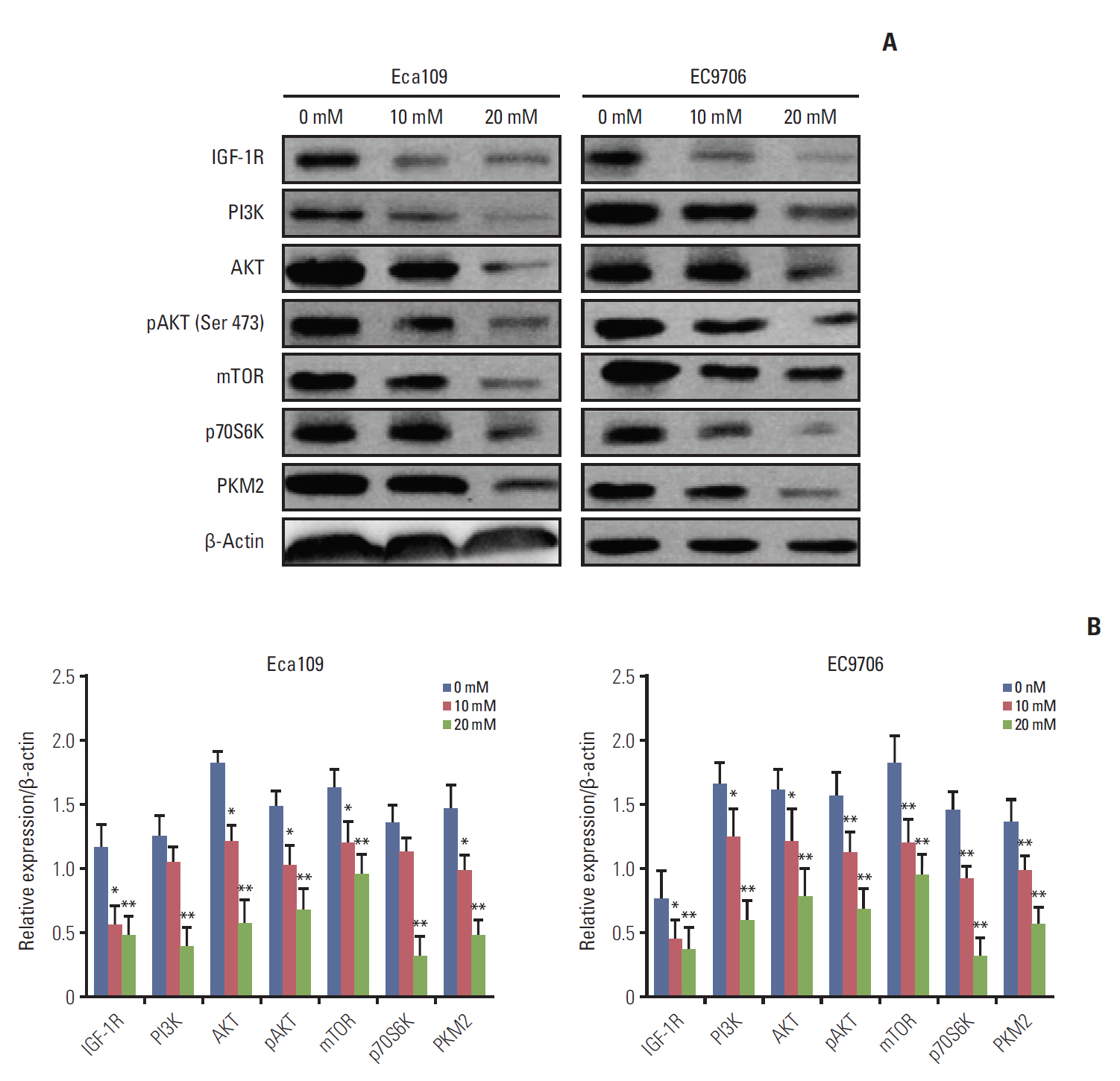
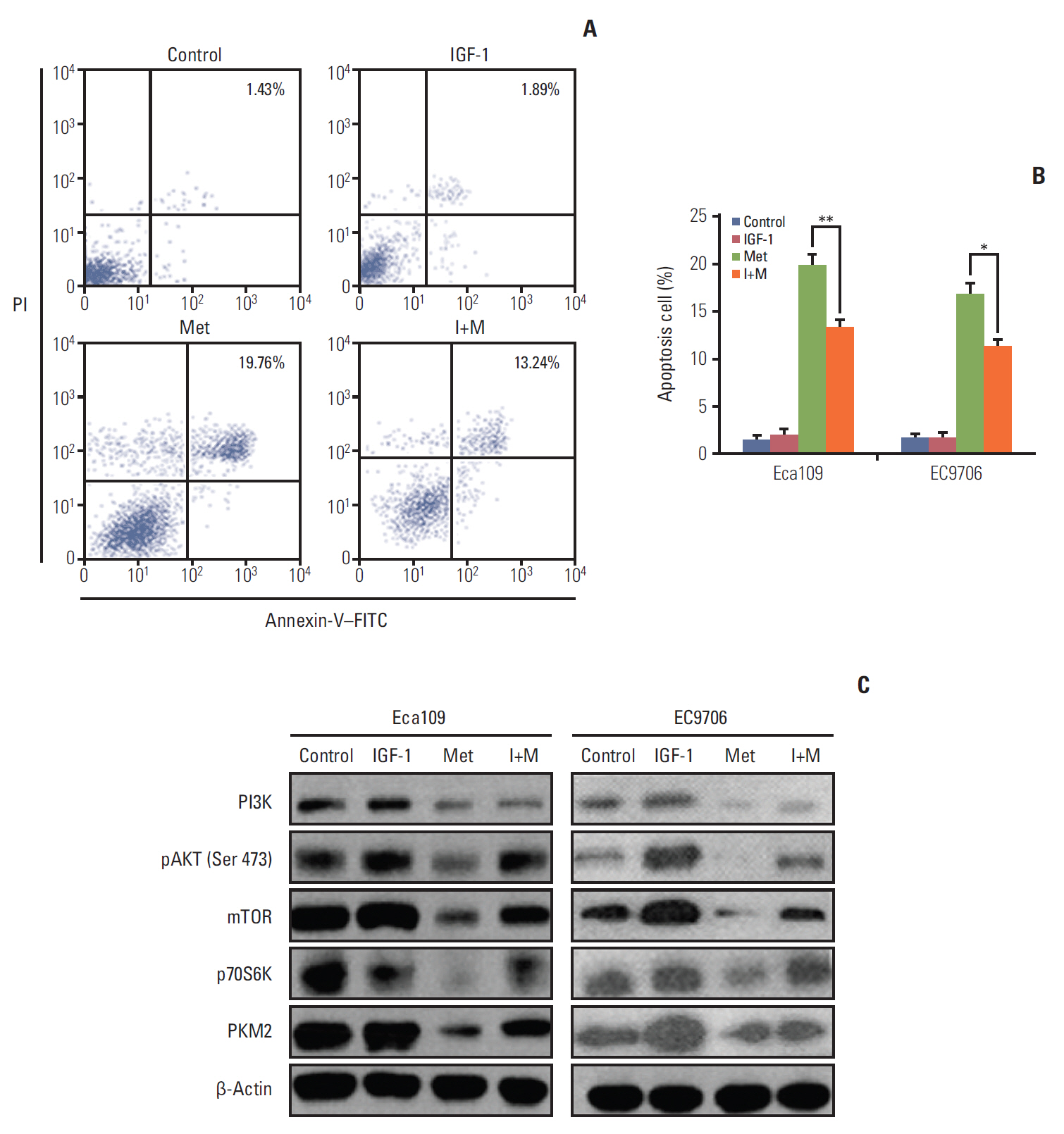
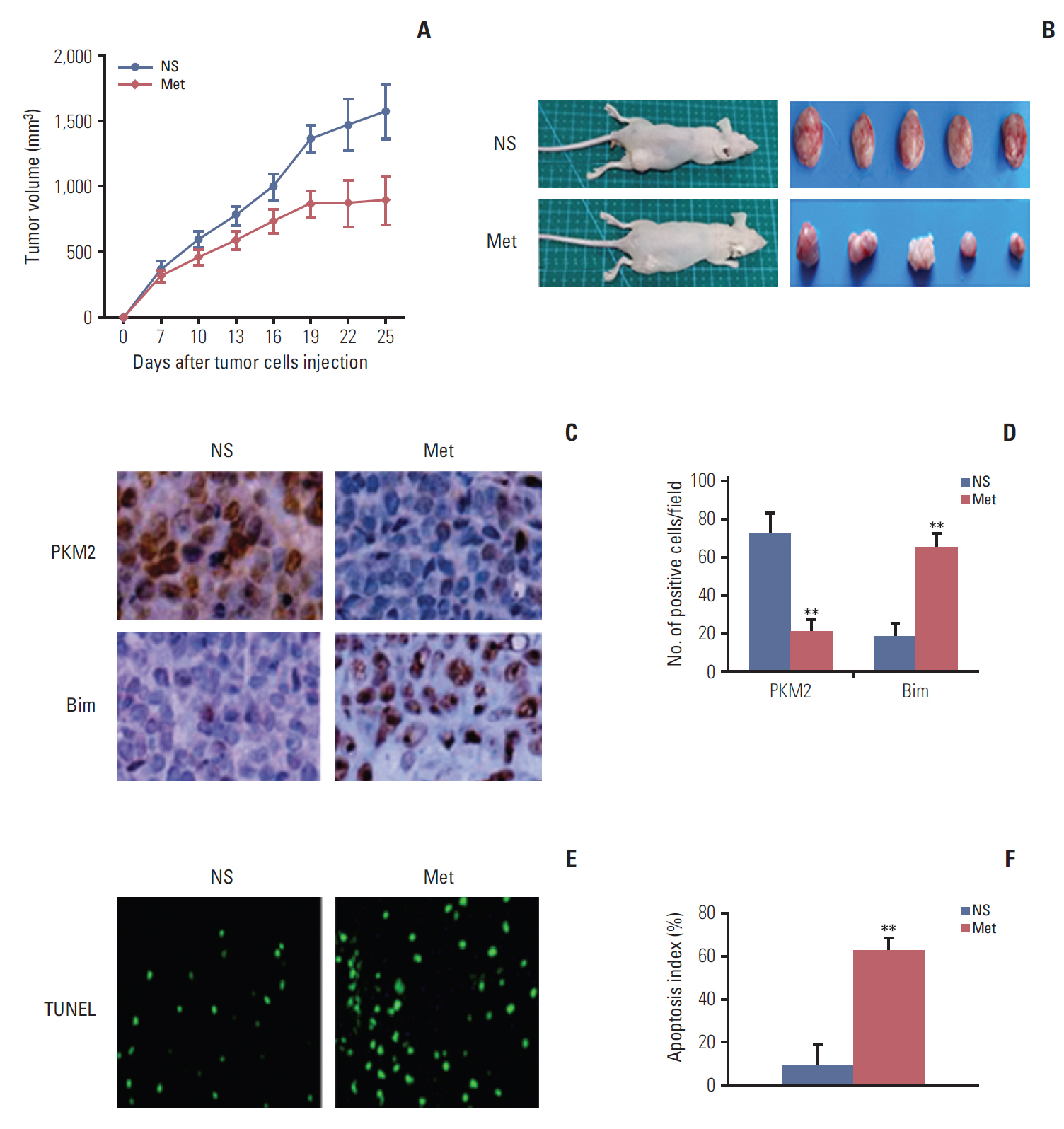
Esophageal cancer cells viability was reduced after metformin treatment. The cell cycle was arrested in the G0/G1 phase, apoptosis was induced, caspase 3 was activated, caspase 9 was downregulated, and the pro-apoptotic protein Bim increased. Further study revealed that metformin could suppress the expression of insulin-like growth factor 1 receptor and its downstream proteins, phosphoinositide 3-kinase (PI3K), protein kinase B (AKT/PKB), phosphorylation of AKT (pAKT), mammalian target of rapamycin (mTOR), p70S6K, and PKM2. Insulin-like growth factor 1 partly reversed metfromin-induced apoptosis and attenuated the repression effect of metfomin to PI3K, pAKT, and PKM2. Knockout PKM2 resulted in the activation of caspase 3, down-regulation of caspase 9, and increased expression of Bim. In the Eca109 xenograft model, metformin significantly reduced tumor growth. Furthermore, we found that metformin treatment increased the rate of apoptosis, down-regulation of PKM2, and up-regulation of Bim in tumor tissues.
CONCLUSION
Metformin restrained esophageal cancer cell proliferation partly by suppressing the PI3K/AKT/mTOR pathway.
Keyword
MeSH Terms
-
Apoptosis
Blotting, Western
Caspase 3
Caspase 9
Cell Count
Cell Cycle
Cell Proliferation
Cell Survival
Down-Regulation
Esophageal Neoplasms*
Flow Cytometry
Heterografts
Humans
Immunohistochemistry
In Vitro Techniques*
Metformin*
Phosphorylation
Proto-Oncogene Proteins c-akt
Pyruvate Kinase
Repression, Psychology
Ribosomal Protein S6 Kinases, 70-kDa
RNA Interference
Sirolimus
Up-Regulation
Caspase 3
Caspase 9
Metformin
Proto-Oncogene Proteins c-akt
Pyruvate Kinase
Ribosomal Protein S6 Kinases, 70-kDa
Sirolimus
Figure
Reference
-
References
1. Zhuang Y, Miskimins WK. Cell cycle arrest in Metformin treated breast cancer cells involves activation of AMPK, down-regulation of cyclin D1, and requires p27Kip1 or p21Cip1. J Mol Signal. 2008; 3:18.2. Huang Y, Guo W, Shi S, He J. Evaluation of the 7(th) edition of the UICC-AJCC tumor, node, metastasis classification for esophageal cancer in a Chinese cohort. J Thorac Dis. 2016; 8:1672–80.
Article3. Xie YE, Tang EJ, Zhang DR, Ren BX. Down-regulation of BclXL by RNA interference suppresses cell growth and induces apoptosis in human esophageal cancer cells. World J Gastroenterol. 2006; 12:7472–7.4. Tan R, Yao SZ, Huang ZQ, Li J, Li X, Tan HH, et al. Combination of FDG PET/CT and contrast-enhanced MSCT in detecting lymph node metastasis of esophageal cancer. Asian Pac J Cancer Prev. 2014; 15:7719–24.
Article5. Kato K, Gong J, Iwama H, Kitanaka A, Tani J, Miyoshi H, et al. The antidiabetic drug metformin inhibits gastric cancer cell proliferation in vitro and in vivo. Mol Cancer Ther. 2012; 11:549–60.
Article6. Shi WY, Xiao D, Wang L, Dong LH, Yan ZX, Shen ZX, et al. Therapeutic metformin/AMPK activation blocked lymphoma cell growth via inhibition of mTOR pathway and induction of autophagy. Cell Death Dis. 2012; 3:e275.
Article7. Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006; 66:10269–73.
Article8. Klil-Drori AJ, Azoulay L, Pollak MN. Cancer, obesity, diabetes, and antidiabetic drugs: is the fog clearing? Nat Rev Clin Oncol. 2017; 14:85–99.
Article9. Mu L, Zhu N, Zhang J, Xing F, Li D, Wang X. Type 2 diabetes, insulin treatment and prognosis of breast cancer. Diabetes Metab Res Rev. 2017; 33:e2823.
Article10. Vigneri R, Frasca F, Sciacca L, Vigneri P, Frittitta L. Re: Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009; 101:1030–1.
Article11. Skinner HD, McCurdy MR, Echeverria AE, Lin SH, Welsh JW, O'Reilly MS, et al. Metformin use and improved response to therapy in esophageal adenocarcinoma. Acta Oncol. 2013; 52:1002–9.
Article12. Wild SH. Diabetes, treatments for diabetes and their effect on cancer incidence and mortality: attempts to disentangle the web of associations. Diabetologia. 2011; 54:1589–92.
Article13. Walker JJ, Johnson JA, Wild SH. Diabetes treatments and cancer risk: the importance of considering aspects of drug exposure. Lancet Diabetes Endocrinol. 2013; 1:132–9.
Article14. Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen-induced lung tumorigenesis. Cancer Prev Res (Phila). 2010; 3:1066–76.
Article15. Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011; 71:3196–201.
Article16. Rocha GZ, Dias MM, Ropelle ER, Osorio-Costa F, Rossato FA, Vercesi AE, et al. Metformin amplifies chemotherapy-induced AMPK activation and antitumoral growth. Clin Cancer Res. 2011; 17:3993–4005.
Article17. Zhuo Z, Wang A, Yu H. Metformin targeting autophagy overcomes progesterone resistance in endometrial carcinoma. Arch Gynecol Obstet. 2016; 294:1055–61.
Article18. Poli G, Cantini G, Armignacco R, Fucci R, Santi R, Canu L, et al. Metformin as a new anti-cancer drug in adrenocortical carcinoma. Oncotarget. 2016; 7:49636–48.
Article19. Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007; 100:328–41.
Article20. Ma W, Zhang T, Pan J, Shi N, Fan Q, Wang L, et al. Assessment of insulin-like growth factor 1 receptor as an oncogene in esophageal squamous cell carcinoma and its potential implication in chemotherapy. Oncol Rep. 2014; 32:1601–9.
Article21. Imsumran A, Adachi Y, Yamamoto H, Li R, Wang Y, Min Y, et al. Insulin-like growth factor-I receptor as a marker for prognosis and a therapeutic target in human esophageal squamous cell carcinoma. Carcinogenesis. 2007; 28:947–56.
Article22. Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008; 452:230–3.
Article23. Harris I, McCracken S, Mak TW. PKM2: a gatekeeper between growth and survival. Cell Res. 2012; 22:447–9.
Article24. Jain M, Nilsson R, Sharma S, Madhusudhan N, Kitami T, Souza AL, et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012; 336:1040–4.
Article25. Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, et al. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell. 2012; 150:685–96.
Article26. Cai X, Hu X, Tan X, Cheng W, Wang Q, Chen X, et al. Metformin induced AMPK activation, G0/G1 phase cell cycle arrest and the inhibition of growth of esophageal squamous cell carcinomas in vitro and in vivo. PLoS One. 2015; 10:e0133349.
Article27. Banerjee J, Bruckbauer A, Zemel MB. Activation of the AMPK/Sirt1 pathway by a leucine-metformin combination increases insulin sensitivity in skeletal muscle, and stimulates glucose and lipid metabolism and increases life span in Caenorhabditis elegans. Metabolism. 2016; 65:1679–91.
Article28. Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov. 2012; 2:778–90.
Article29. Munoz-Pinedo C, El Mjiyad N, Ricci JE. Cancer metabolism: current perspectives and future directions. Cell Death Dis. 2012; 3:e248.
Article30. Feng Y, Ke C, Tang Q, Dong H, Zheng X, Lin W, et al. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis. 2014; 5:e1088.
Article31. Ye P, Qu CF, Hu XL. Impact of IGF-1, IGF-1R, and IGFBP-3 promoter methylation on the risk and prognosis of esophageal carcinoma. Tumour Biol. 2016; 37:6893–904.
Article32. Iqbal MA, Gupta V, Gopinath P, Mazurek S, Bamezai RN. Pyruvate kinase M2 and cancer: an updated assessment. FEBS Lett. 2014; 588:2685–92.
Article33. Cheong JH, Park ES, Liang J, Dennison JB, Tsavachidou D, Nguyen-Charles C, et al. Dual inhibition of tumor energy pathway by 2-deoxyglucose and metformin is effective against a broad spectrum of preclinical cancer models. Mol Cancer Ther. 2011; 10:2350–62.
Article34. Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010; 33:1674–85.
Article35. Silvestri A, Palumbo F, Rasi I, Posca D, Pavlidou T, Paoluzi S, et al. Metformin induces apoptosis and downregulates pyruvate kinase M2 in breast cancer cells only when grown in nutrient-poor conditions. PLoS One. 2015; 10:e0136250.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metformin displays in vitro and in vivo antitumor effect against osteosarcoma
- Sanghuangporus sanghuang extract inhibits the proliferation and invasion of lung cancer cells in vitro and in vivo
- Metformin Suppresses Both PD-L1 Expression in Cancer Cells and Cancer-Induced PD-1 Expression in Immune Cells to Promote Antitumor Immunity
- Rethinking about Metformin: Promising Potentials
- The Metformin Use and Gastric Cancer Risk