Ann Lab Med.
2024 Sep;44(5):426-436. 10.3343/alm.2023.0443.
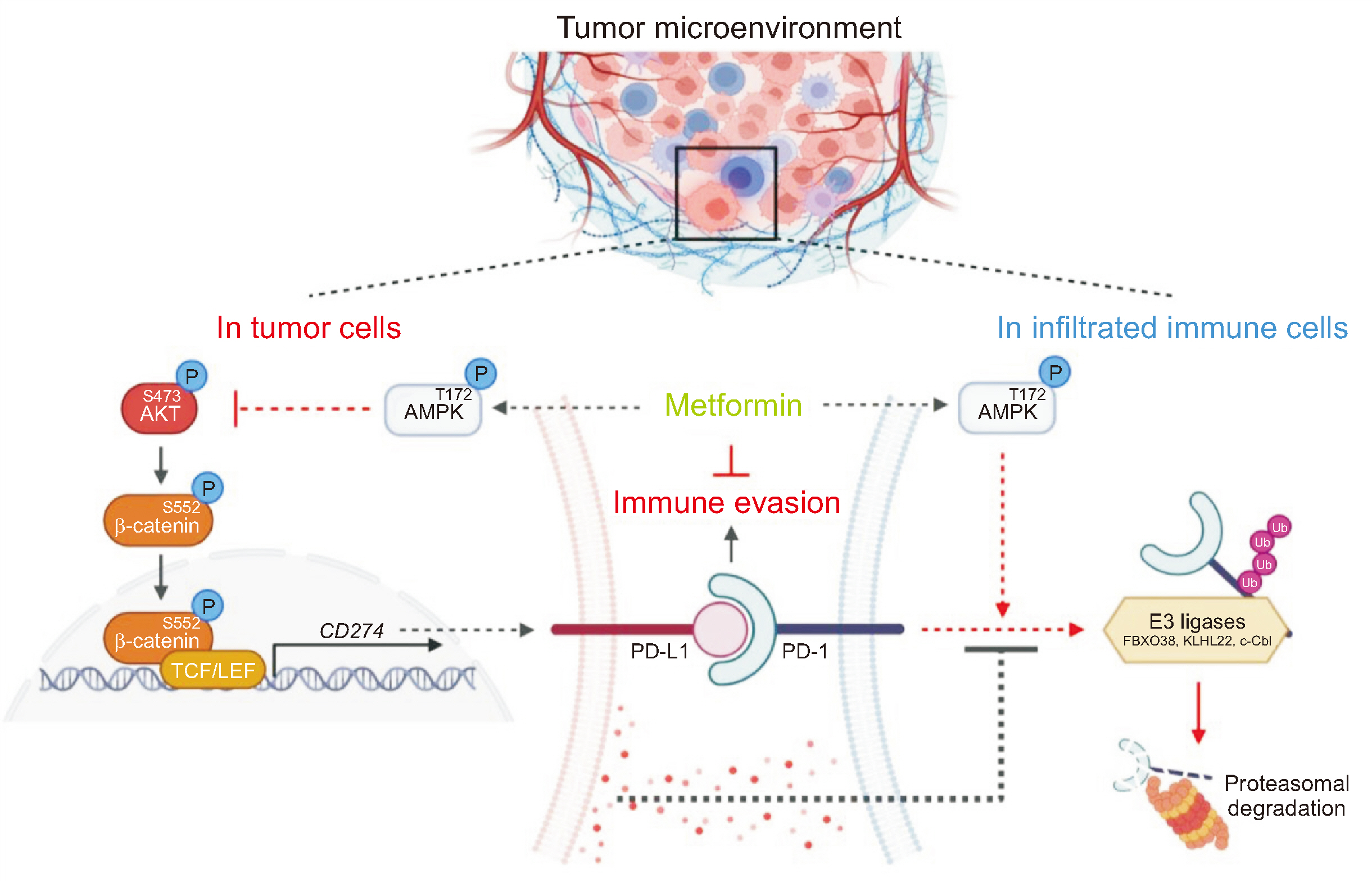
Metformin Suppresses Both PD-L1 Expression in Cancer Cells and Cancer-Induced PD-1 Expression in Immune Cells to Promote Antitumor Immunity
- Affiliations
-
- 1Department of Health Sciences, The Graduate School of Dong-A University, Busan, Korea
- 2Department of Medicinal Biotechnology, College of Health Science, Dong-A University, Busan, Korea
- 3Department of Biomedical Sciences, Dong-A University, Busan, Korea
- KMID: 2559149
- DOI: http://doi.org/10.3343/alm.2023.0443
Abstract
- Background
Metformin, a drug prescribed for patients with type 2 diabetes, has potential efficacy in enhancing antitumor immunity; however, the detailed underlying mechanisms remain to be elucidated. Therefore, we aimed to identify the inhibitory molecular mechanisms of metformin on programmed death ligand 1 (PD-L1) expression in cancer cells and programmed death 1 (PD-1) expression in immune cells.
Methods
We employed a luciferase reporter assay, quantitative real-time PCR, immunoblotting analysis, immunoprecipitation and ubiquitylation assays, and a natural killer (NK) cell-mediated tumor cell cytotoxicity assay. A mouse xenograft tumor model was used to evaluate the effect of metformin on tumor growth, followed by flow-cytometric analysis using tumor-derived single-cell suspensions.
Results
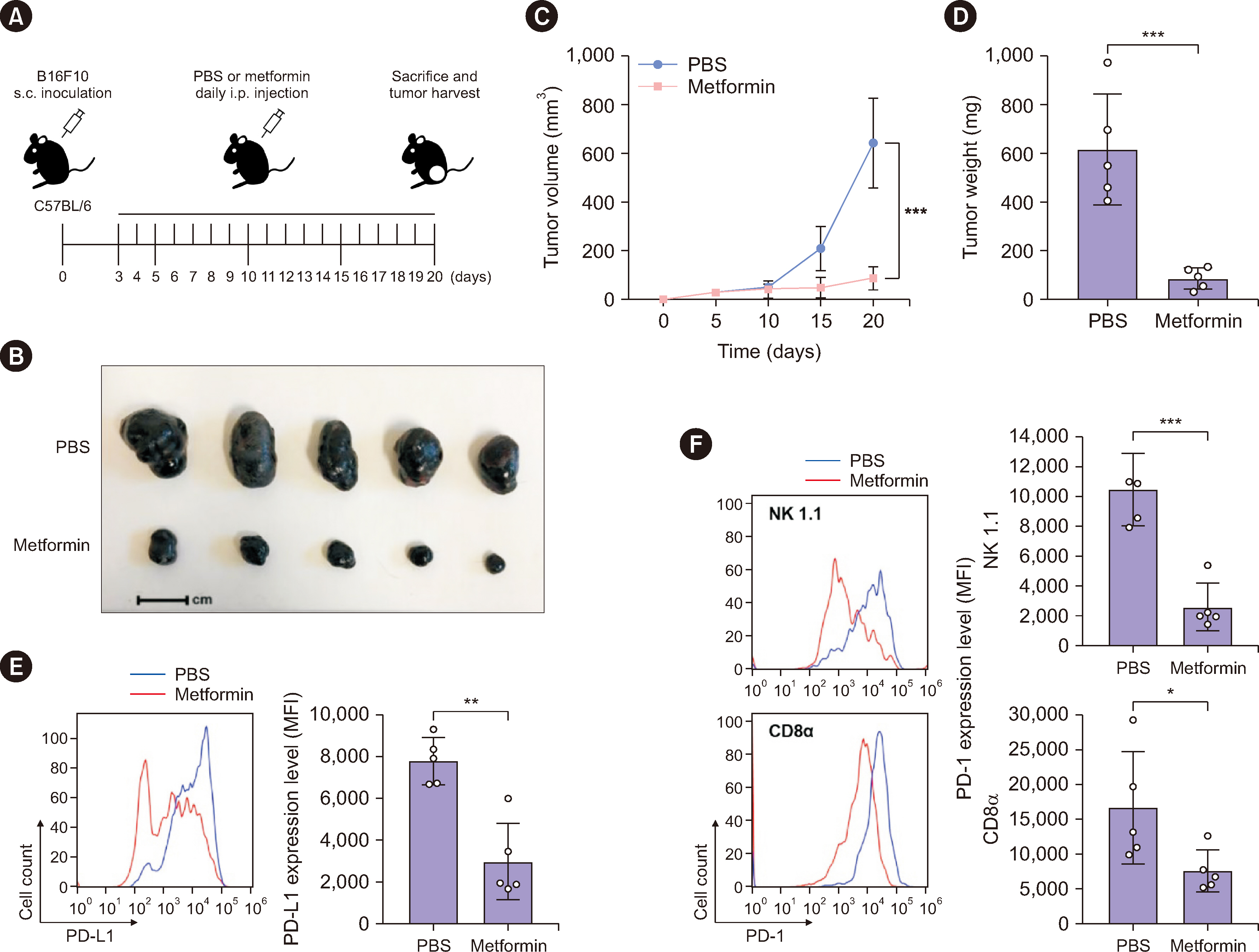
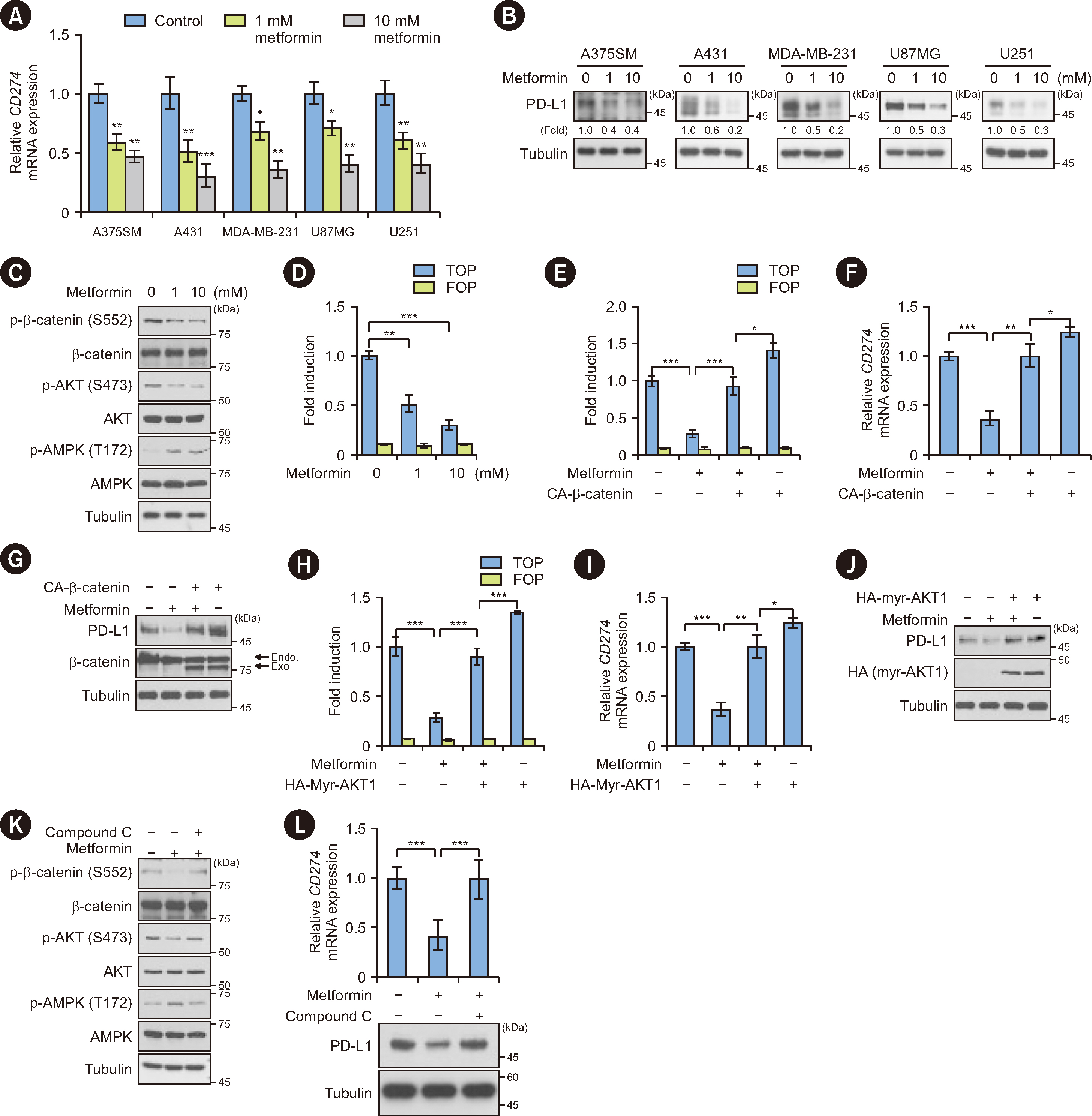
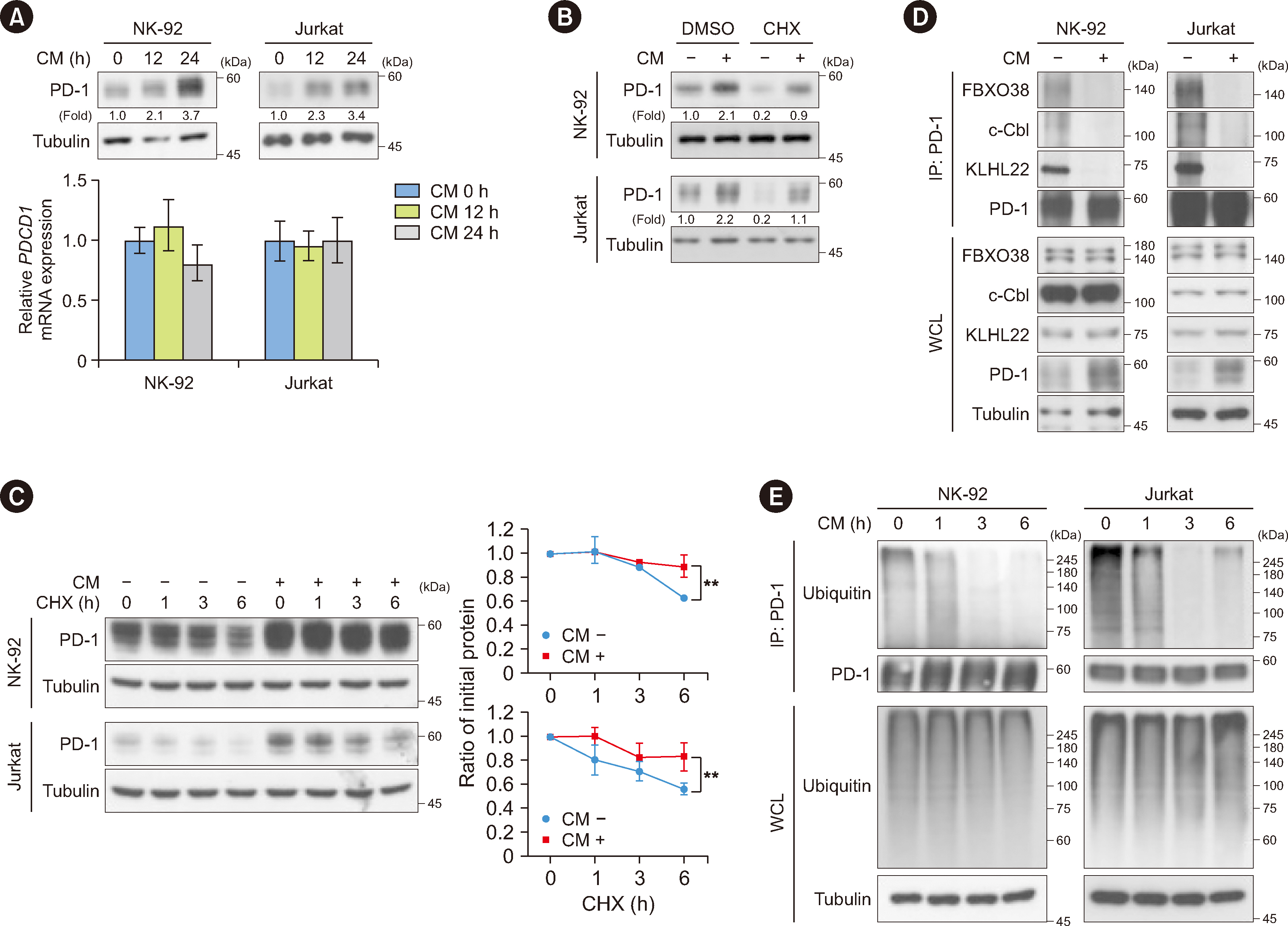
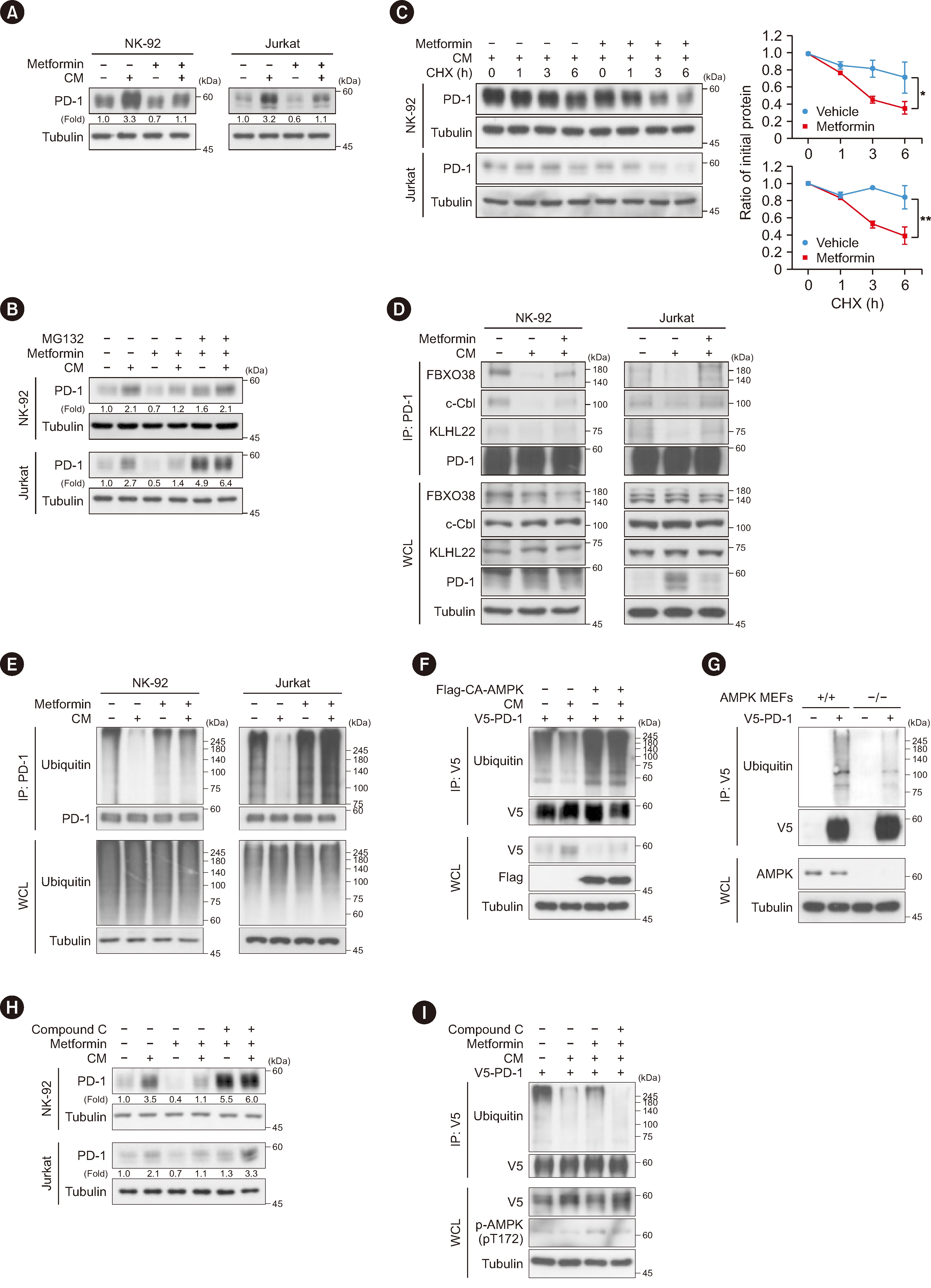
Metformin decreased AKT-mediated β-catenin S552 phosphorylation and subsequent β-catenin transactivation in an adenosine monophosphate-activated protein kinase (AMPK) activation-dependent manner, resulting in reduced CD274 (encoding PD-L1) transcription in cancer cells. Tumor-derived soluble factors enhanced PD-1 protein stability in NK and T cells via dissociation of PD-1 from ubiquitin E3 ligases and reducing PD-1 polyubiquitylation. Metformin inhibited the tumor-derived soluble factor-reduced binding of PD-1 to E3 ligases and PD-1 polyubiquitylation, resulting in PD-1 protein downregulation in an AMPK activation-dependent manner. These inhibitory effects of metformin on both PD-L1 and PD-1 expression ameliorated cancer-reduced cytotoxic activity of immune cells in vitro and decreased tumor immune evasion and growth in vivo.
Conclusions
Metformin blocks both PD-L1 and PD-1 within the tumor microenvironment. This study provided a mechanistic insight into the efficacy of metformin in improving immunotherapy in human cancer.
Keyword
Figure
Reference
-
References
1. Boussiotis VA. 2016; Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 375:1767–78. DOI: 10.1056/NEJMra1514296. PMID: 27806234. PMCID: PMC5575761.
Article2. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. 2017; Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 168:707–23. DOI: 10.1016/j.cell.2017.01.017. PMID: 28187290. PMCID: PMC5391692.
Article3. Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, et al. 2019; Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 18:10. DOI: 10.1186/s12943-018-0928-4. PMID: 30646912. PMCID: PMC6332843. PMID: 70238db4d68c4e4bb58930633279c9c3.
Article4. Du L, Lee JH, Jiang H, Wang C, Wang S, Zheng Z, et al. 2020; β-Catenin induces transcriptional expression of PD-L1 to promote glioblastoma immune evasion. J Exp Med. 217:e20191115. DOI: 10.1084/jem.20191115. PMID: 32860047. PMCID: PMC7596815.
Article5. Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. 2007; Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 104:3360–5. DOI: 10.1073/pnas.0611533104. PMID: 17360651. PMCID: PMC1805580.6. Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. 2004; Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 101:17174–9. DOI: 10.1073/pnas.0406351101. PMID: 15569934. PMCID: PMC534606.
Article7. Nusse R, Clevers H. 2017; Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 169:985–99. DOI: 10.1016/j.cell.2017.05.016. PMID: 28575679.
Article8. Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. 2009; Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 114:1537–44. DOI: 10.1182/blood-2008-12-195792. PMID: 19423728. PMCID: PMC2927090.
Article9. Pesce S, Greppi M, Tabellini G, Rampinelli F, Parolini S, Olive D, et al. 2017; Identification of a subset of human natural killer cells expressing high levels of programmed death 1: a phenotypic and functional characterization. J Allergy Clin Immunol. 139:335–46.e3. DOI: 10.1016/j.jaci.2016.04.025. PMID: 27372564.10. Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, et al. 2018; Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest. 128:4654–68. DOI: 10.1172/JCI99317. PMID: 30198904. PMCID: PMC6159991.
Article11. Muti P, Berrino F, Krogh V, Villarini A, Barba M, Strano S, et al. 2009; Metformin, diet and breast cancer: an avenue for chemoprevention. Cell Cycle. 8:2661. DOI: 10.4161/cc.8.16.9226. PMID: 19571669.
Article12. Vancura A, Bu P, Bhagwat M, Zeng J, Vancurova I. 2018; Metformin as an anticancer agent. Trends Pharmacol Sci. 39:867–78. DOI: 10.1016/j.tips.2018.07.006. PMID: 30150001. PMCID: PMC6153060.
Article13. Wink KCJ, Belderbos JSA, Dieleman EMT, Rossi M, Rasch CRN, Damhuis RAM, et al. 2016; Improved progression free survival for patients with diabetes and locally advanced non-small cell lung cancer (NSCLC) using metformin during concurrent chemoradiotherapy. Radiother Oncol. 118:453–9. DOI: 10.1016/j.radonc.2016.01.012. PMID: 26861738.
Article14. Saif MW, Rajagopal S, Caplain J, Grimm E, Serebrennikova O, Das M, et al. 2019; A phase I delayed-start, randomized and pharmacodynamic study of metformin and chemotherapy in patients with solid tumors. Cancer Chemother Pharmacol. 84:1323–31. DOI: 10.1007/s00280-019-03967-3. PMID: 31583436.
Article15. Iliopoulos D, Hirsch HA, Struhl K. 2011; Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 71:3196–201. DOI: 10.1158/0008-5472.CAN-10-3471. PMID: 21415163. PMCID: PMC3085572.
Article16. Zhang CS, Li M, Ma T, Zong Y, Cui J, Feng JW, et al. 2016; Metformin activates AMPK through the lysosomal pathway. Cell Metab. 24:521–2. DOI: 10.1016/j.cmet.2016.09.003. PMID: 27732831.
Article17. Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. 2007; Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 67:10804–12. DOI: 10.1158/0008-5472.CAN-07-2310. PMID: 18006825.
Article18. Blandino G, Valerio M, Cioce M, Mori F, Casadei L, Pulito C, et al. 2012; Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat Commun. 3:865. DOI: 10.1038/ncomms1859. PMID: 22643892.
Article19. Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, et al. 2011; Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 71:4366–72. DOI: 10.1158/0008-5472.CAN-10-1769. PMID: 21540236.
Article20. Cha JH, Yang WH, Xia W, Wei Y, Chan LC, Lim SO, et al. 2018; Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Mol Cell. 71:606–20.e7. DOI: 10.1016/j.molcel.2018.07.030. PMID: 30118680. PMCID: PMC6786495.
Article21. Lu Y, Xin D, Guan L, Xu M, Yang Y, Chen Y, et al. 2021; Metformin downregulates PD-L1 expression in esophageal squamous cell carcinoma by inhibiting IL-6 signaling pathway. Front Oncol. 11:762523. DOI: 10.3389/fonc.2021.762523. PMID: 34881181. PMCID: PMC8645640. PMID: c861b8c7f13c4c35a4f101a9ac29c246.
Article22. Zhang JJ, Zhang QS, Li ZQ, Zhou JW, Du J. 2019; Metformin attenuates PD-L1 expression through activating Hippo signaling pathway in colorectal cancer cells. Am J Transl Res. 11:6965–76. PMID: 31814900. PMCID: PMC6895520.23. Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W, et al. 2011; Nuclear PKM2 regulates beta-catenin transactivation upon EGFR activation. Nature. 480:118–22. DOI: 10.1038/nature10598. PMID: 22056988. PMCID: PMC3235705.
Article24. Rattan R, Giri S, Singh AK, Singh I. 2005; 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J Biol Chem. 280:39582–93. DOI: 10.1074/jbc.M507443200. PMID: 16176927.25. Russo V, Protti MP. 2017; Tumor-derived factors affecting immune cells. Cytokine Growth Factor Rev. 36:79–87. DOI: 10.1016/j.cytogfr.2017.06.005. PMID: 28606733.
Article26. Meng X, Liu X, Guo X, Jiang S, Chen T, Hu Z, et al. 2018; FBXO38 mediates PD-1 ubiquitination and regulates anti-tumour immunity of T cells. Nature. 564:130–5. DOI: 10.1038/s41586-018-0756-0. PMID: 30487606.
Article27. Lyle C, Richards S, Yasuda K, Napoleon MA, Walker J, Arinze N, et al. 2019; c-Cbl targets PD-1 in immune cells for proteasomal degradation and modulates colorectal tumor growth. Sci Rep. 9:20257. DOI: 10.1038/s41598-019-56208-1. PMID: 31882749. PMCID: PMC6934810.
Article28. Zhou XA, Zhou J, Zhao L, Yu G, Zhan J, Shi C, et al. 2020; KLHL22 maintains PD-1 homeostasis and prevents excessive T cell suppression. Proc Natl Acad Sci U S A. 117:28239–50. DOI: 10.1073/pnas.2004570117. PMID: 33109719. PMCID: PMC7668036.
Article29. Kubo T, Ninomiya T, Hotta K, Kozuki T, Toyooka S, Okada H, et al. 2018; Study protocol: phase-Ib trial of nivolumab combined with metformin for refractory/recurrent solid tumors. Clin Lung Cancer. 19:e861–4. DOI: 10.1016/j.cllc.2018.07.010. PMID: 30172698.
Article30. Oestreich KJ, Yoon H, Ahmed R, Boss JM. 2008; NFATc1 regulates PD-1 expression upon T cell activation. J Immunol. 181:4832–9. DOI: 10.4049/jimmunol.181.7.4832. PMID: 18802087. PMCID: PMC2645436.
Article31. Austin JW, Lu P, Majumder P, Ahmed R, Boss JM. 2014; STAT3, STAT4, NFATc1, and CTCF regulate PD-1 through multiple novel regulatory regions in murine T cells. J Immunol. 192:4876–86. DOI: 10.4049/jimmunol.1302750. PMID: 24711622. PMCID: PMC4011967.
Article32. Cho HY, Lee SW, Seo SK, Choi IW, Choi I, Lee SW. 2008; Interferon-sensitive response element (ISRE) is mainly responsible for IFN-alpha-induced upregulation of programmed death-1 (PD-1) in macrophages. Biochim Biophys Acta. 1779:811–9. DOI: 10.1016/j.bbagrm.2008.08.003. PMID: 18771758.33. Terawaki S, Chikuma S, Shibayama S, Hayashi T, Yoshida T, Okazaki T, et al. 2011; IFN-α directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J Immunol. 186:2772–9. DOI: 10.4049/jimmunol.1003208. PMID: 21263073.
Article34. Tie Y, Tang F, Wei YQ, Wei XW. 2022; Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J Hematol Oncol. 15:61. DOI: 10.1186/s13045-022-01282-8. PMID: 35585567. PMCID: PMC9118588. PMID: 274a447483bf40e79ac6103100cdf0e6.
Article35. Wang S, Lin Y, Xiong X, Wang L, Guo Y, Chen Y, et al. 2020; Low-dose metformin reprograms the tumor immune microenvironment in human esophageal cancer: results of a phase II clinical trial. Clin Cancer Res. 26:4921–32. DOI: 10.1158/1078-0432.CCR-20-0113. PMID: 32646922.
Article36. Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. 2015; Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A. 112:1809–14. DOI: 10.1073/pnas.1417636112. PMID: 25624476. PMCID: PMC4330733.
Article37. Crist M, Yaniv B, Palackdharry S, Lehn MA, Medvedovic M, Stone T, et al. 2022; Metformin increases natural killer cell functions in head and neck squamous cell carcinoma through CXCL1 inhibition. J Immunother Cancer. 10:e005632. DOI: 10.1136/jitc-2022-005632. PMID: 36328378. PMCID: PMC9639146. PMID: d5e39b30e67c484baead80341faf7c76.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of Programmed Death-Ligand 1 on Immune Cells Is Related to a Better Prognosis in Biliary Tract Cancer
- PD-L1 Expression and Combined Status of PD-L1/PD-1–Positive Tumor Infiltrating Mononuclear Cell Density Predict Prognosis in Glioblastoma Patients
- Analysis of the Expression and Regulation of PD-1 Protein on the Surface of Myeloid-Derived Suppressor Cells (MDSCs)
- PD-1: A Negative Regulator of Phagocytosis by Tumour-Associated Macrophages in Colon Cancer
- PD-L1 expression correlated with p53 expression in oral squamous cell carcinoma