J Pathol Transl Med.
2017 Jan;51(1):40-48. 10.4132/jptm.2016.08.31.
PD-L1 Expression and Combined Status of PD-L1/PD-1–Positive Tumor Infiltrating Mononuclear Cell Density Predict Prognosis in Glioblastoma Patients
- Affiliations
-
- 1Department of Hospital Pathology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. lys9908@catholic.ac.kr
- 2Department of Neurosurgery, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2367679
- DOI: http://doi.org/10.4132/jptm.2016.08.31
Abstract
- BACKGROUND
Programmed death ligand 1 (PD-L1) in tumor cells is known to promote immune escape of cancer by interacting with programmed cell death 1 (PD-1) in tumor infiltrating immune cells. Immunotherapy targeting these molecules is emerging as a new strategy for the treatment of glioblastoma (GBM). Understanding the relationship between the PD-L1/PD-1 axis and prognosis in GBM patients may be helpful to predict the effects of immunotherapy.
METHODS
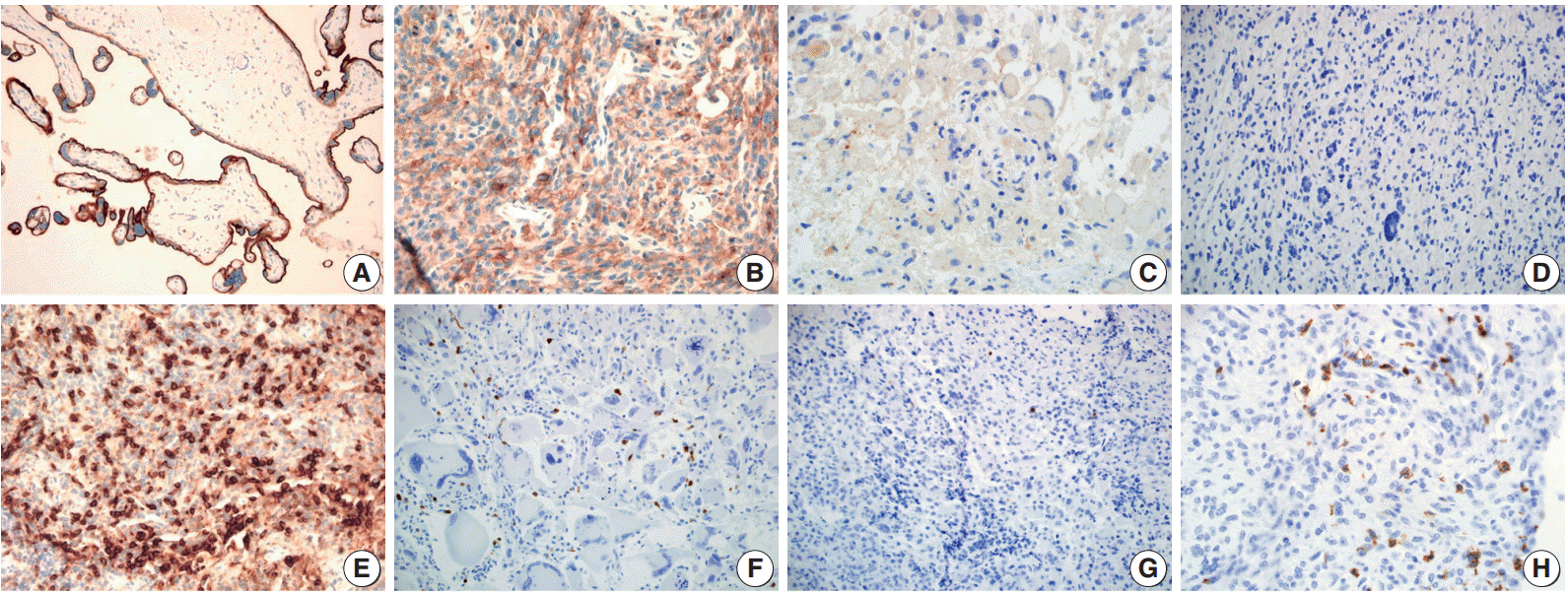
PD-L1 expression and PD-1-positive tumor infiltrating mononuclear cell (PD-1+tumor infiltrating mononuclear cell [TIMC]) density were evaluated using tissue microarray containing 54 GBM cases by immunohistochemical analysis; the associations with patient clinical outcomes were evaluated.
RESULTS
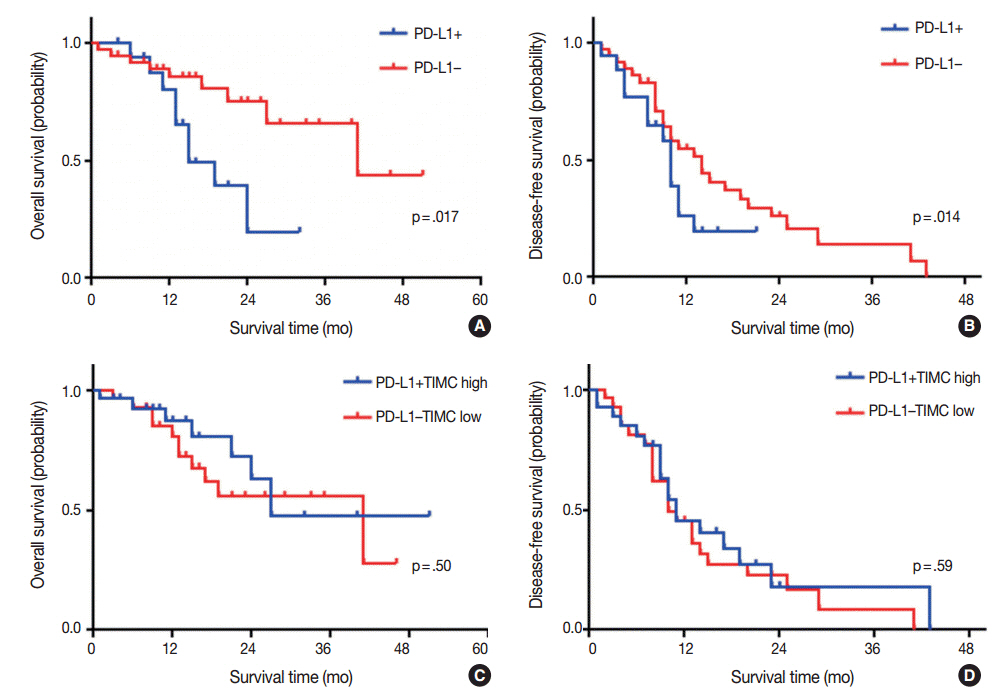
PD-L1 expression and high PD-1+TIMC density were observed in 31.5% and 50% of GBM cases, respectively. High expression of PD-L1 in tumor cells was an independent and significant predictive factor for worse overall survival (OS; hazard ratio, 4.958; p = .007) but was not a significant factor in disease-free survival (DFS). PD-1+TIMC density was not correlated with OS or DFS. When patients were classified based on PD-1 expression and PD-1+TIMC density, patients with PD-L1+/PD-1+TIMC low status had the shortest OS (13 months, p = .009) and DFS (7 months, p = .053).
CONCLUSIONS
PD-L1 expression in GBM was an independent prognostic factor for poor OS. In addition, combined status of PD-L1 expression and PD-1+TIMC density also predicted patient outcomes, suggesting that the therapeutic role of the PD-1/PD-L1 axis should be considered in the context of GBM immunity.
MeSH Terms
Figure
Cited by 1 articles
-
Programmed Death-Ligand 1 Expression and Its Correlation with Lymph Node Metastasis in Papillary Thyroid Carcinoma
Hyo Jung An, Gyung Hyuck Ko, Jeong-Hee Lee, Jong Sil Lee, Dong Chul Kim, Jung Wook Yang, Min Hye Kim, Jin Pyeong Kim, Eun Jung Jung, Dae Hyun Song
J Pathol Transl Med. 2018;52(1):9-13. doi: 10.4132/jptm.2017.07.26.
Reference
-
1. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008; 359:492–507.
Article2. Weller M, van den Bent M, Hopkins K, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014; 15:e395–403.
Article3. Thomas RP, Xu LW, Lober RM, Li G, Nagpal S. The incidence and significance of multiple lesions in glioblastoma. J Neurooncol. 2013; 112:91–7.
Article4. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013; 39:1–10.
Article5. Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigenspecific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009; 114:1537–44.
Article6. Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007; 19:813–24.
Article7. Berghoff AS, Kiesel B, Widhalm G, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015; 17:1064–75.
Article8. Nduom EK, Wei J, Yaghi NK, et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016; 18:195–205.
Article9. Sanmamed MF, Chen L. Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation. Cancer J. 2014; 20:256–61.
Article10. Han SJ, Ahn BJ, Waldron JS, et al. Gamma interferon-mediated superinduction of B7-H1 in PTEN-deficient glioblastoma: a paradoxical mechanism of immune evasion. Neuroreport. 2009; 20:1597–602.
Article11. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008; 26:677–704.
Article12. Thompson RH, Dong H, Lohse CM, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007; 13:1757–61.
Article13. Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumorinfiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013; 139:667–76.
Article14. Webb JR, Milne K, Nelson BH. PD-1 and CD103 are widely coexpressed on prognostically favorable intraepithelial CD8 T cells in human ovarian cancer. Cancer Immunol Res. 2015; 3:926–35.
Article15. Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012; 4:127ra37.
Article16. Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015; 75:2139–45.
Article17. Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002; 298:850–4.18. Louis DN, Ohgaki H, Wiestler OD, Cavanee WK. WHO classification of tumours of the central nervous system. Lyon: IARC Press;2007. 4th.19. Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013; 31:4311–8.
Article20. Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014; 94:107–16.
Article21. Droeser RA, Hirt C, Viehl CT, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013; 49:2233–42.
Article22. Tokito T, Azuma K, Kawahara A, et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer. 2016; 55:7–14.
Article23. Zeng J, Zhang XK, Chen HD, Zhong ZH, Wu QL, Lin SX. Expression of programmed cell death-ligand 1 and its correlation with clinical outcomes in gliomas. Oncotarget. 2016; 7:8944–55.
Article24. Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007; 104:3360–5.
Article25. Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009; 15:971–9.
Article26. Wang L, Ma Q, Chen X, Guo K, Li J, Zhang M. Clinical significance of B7-H1 and B7-1 expressions in pancreatic carcinoma. World J Surg. 2010; 34:1059–65.
Article27. Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006; 66:3381–5.
Article28. Wang A, Wang HY, Liu Y, et al. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol. 2015; 41:450–6.
Article29. Liu YX, Wang XS, Wang YF, et al. Prognostic significance of PD-L1 expression in patients with gastric cancer in East Asia: a meta-analysis. Onco Targets Ther. 2016; 9:2649–54.30. Razavi SM, Lee KE, Jin BE, Aujla PS, Gholamin S, Li G. Immune evasion strategies of glioblastoma. Front Surg. 2016; 3:11.
Article31. Kim MY, Koh J, Kim S, Go H, Jeon YK, Chung DH. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer. 2015; 88:24–33.
Article32. Berghoff AS, Ricken G, Widhalm G, et al. Tumour-infiltrating lymphocytes and expression of programmed death ligand 1 (PD-L1) in melanoma brain metastases. Histopathology. 2015; 66:289–99.
Article33. Nduom EK, Weller M, Heimberger AB. Immunosuppressive mechanisms in glioblastoma. Neuro Oncol. 2015; 17 Suppl 7:vii9–vii14.34. Duchnowska R, Peksa R, Radecka B, et al. Immune response in breast cancer brain metastases and their microenvironment: the role of the PD-1/PD-L axis. Breast Cancer Res. 2016; 18:43.
Article35. Harter PN, Bernatz S, Scholz A, et al. Distribution and prognostic relevance of tumor-infiltrating lymphocytes (TILs) and PD-1/PD-L1 immune checkpoints in human brain metastases. Oncotarget. 2015; 6:40836–49.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical expression of programmed death-ligand 1 and CD8 in glioblastomas
- The Expression of Programmed Death-Ligand 1 on Immune Cells Is Related to a Better Prognosis in Biliary Tract Cancer
- PD-L1 as a Biomarker in Gastric Cancer Immunotherapy
- Peripheral blood immune cell-based biomarkers in anti-PD-1/PD-L1 therapy
- Programmed death-ligand 1 expression and tumor-infiltrating lymphocytes in non-small cell lung cancer: association with clinicopathologic parameters