Nutr Res Pract.
2023 Dec;17(6):1070-1083. 10.4162/nrp.2023.17.6.1070.
Sanghuangporus sanghuang extract inhibits the proliferation and invasion of lung cancer cells in vitro and in vivo
- Affiliations
-
- 1Institute of Vegetable Science, Hangzhou Academy of Agricultural Sciences, Hangzhou 310024, China
- 2Cancer Institute of Integrated Traditional Chinese and Western Medicine, Zhejiang Academy of Traditional Chinese Medicine, Tongde Hospital of Zhejiang Province, Hangzhou 310012, China
- KMID: 2548865
- DOI: http://doi.org/10.4162/nrp.2023.17.6.1070
Abstract
- BACKGROUND/OBJECTIVES
Sanghuangporus sanghuang (SS) has various medicinal effects, including anti-inflammation and anticancer activities. Despite the extensive research on SS, its molecular mechanisms of action on lung cancer are unclear. This study examined the impact of an SS alcohol extract (SAE) on lung cancer using in vitro and in vivo models.
MATERIALS/METHODS
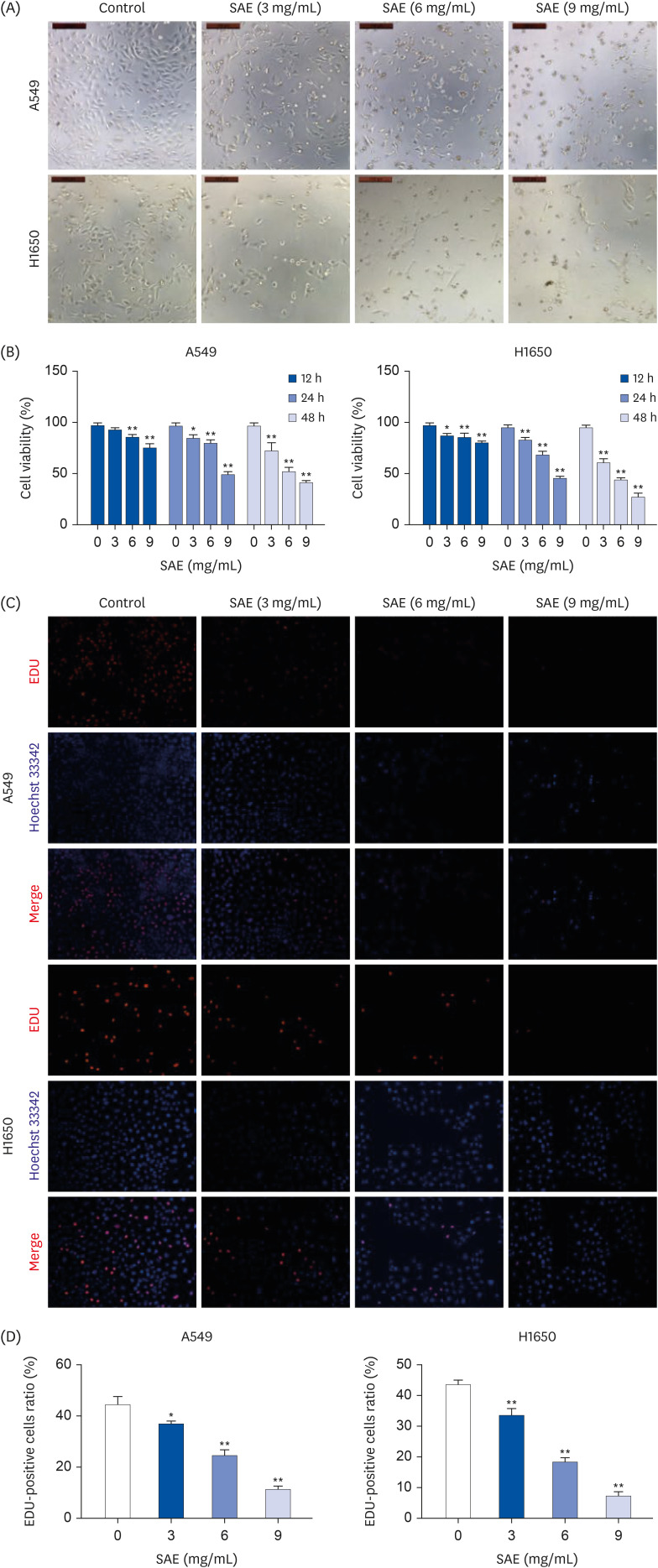
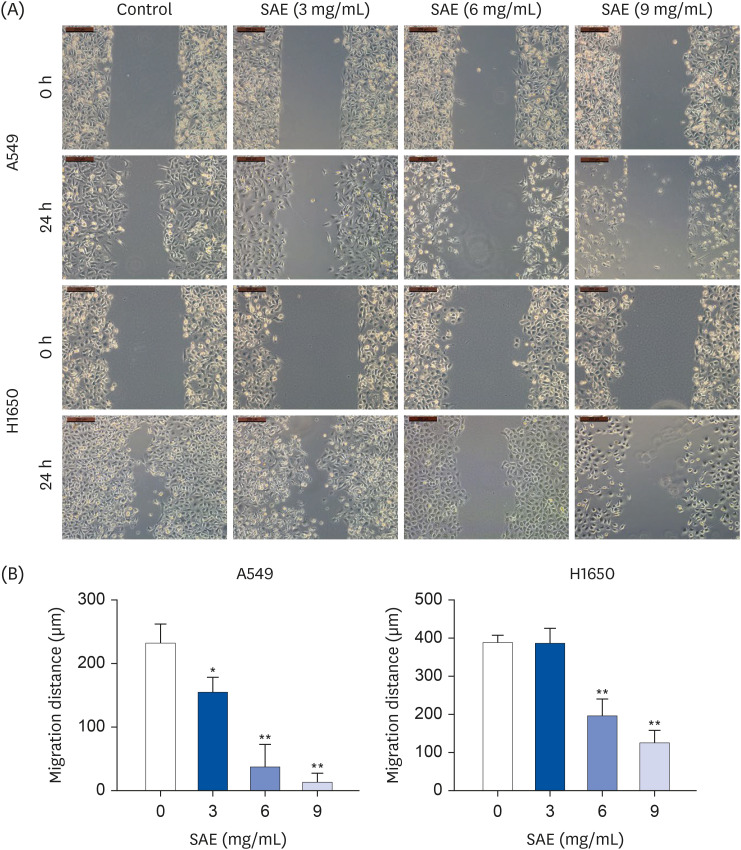
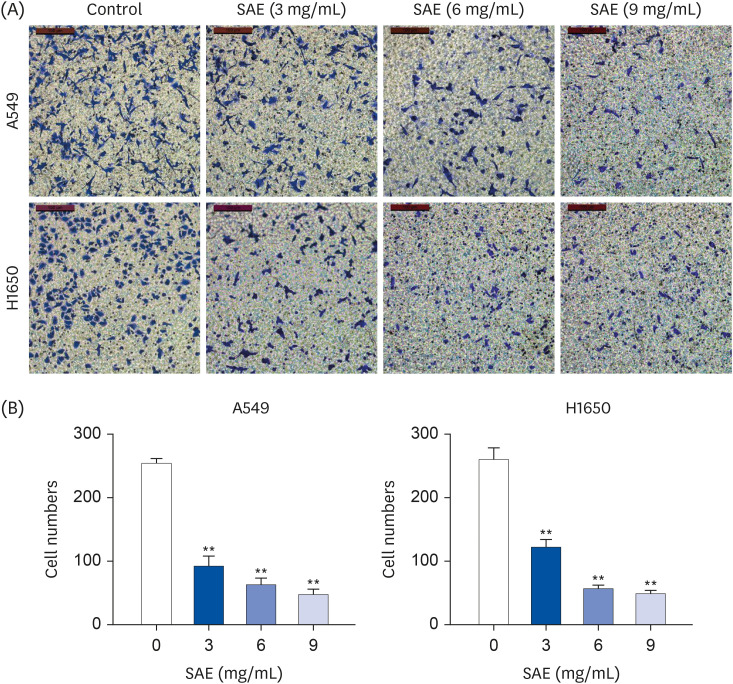
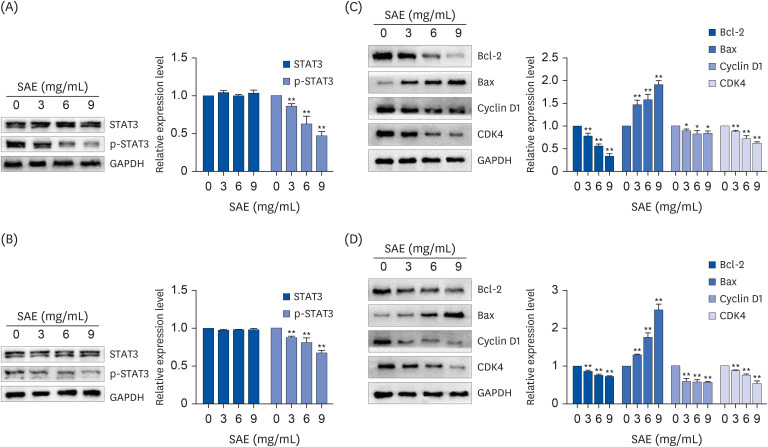
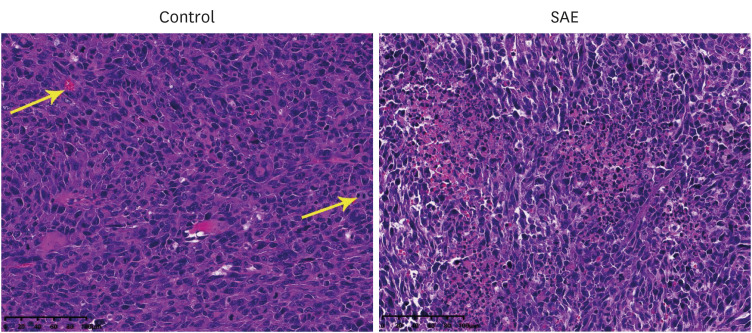
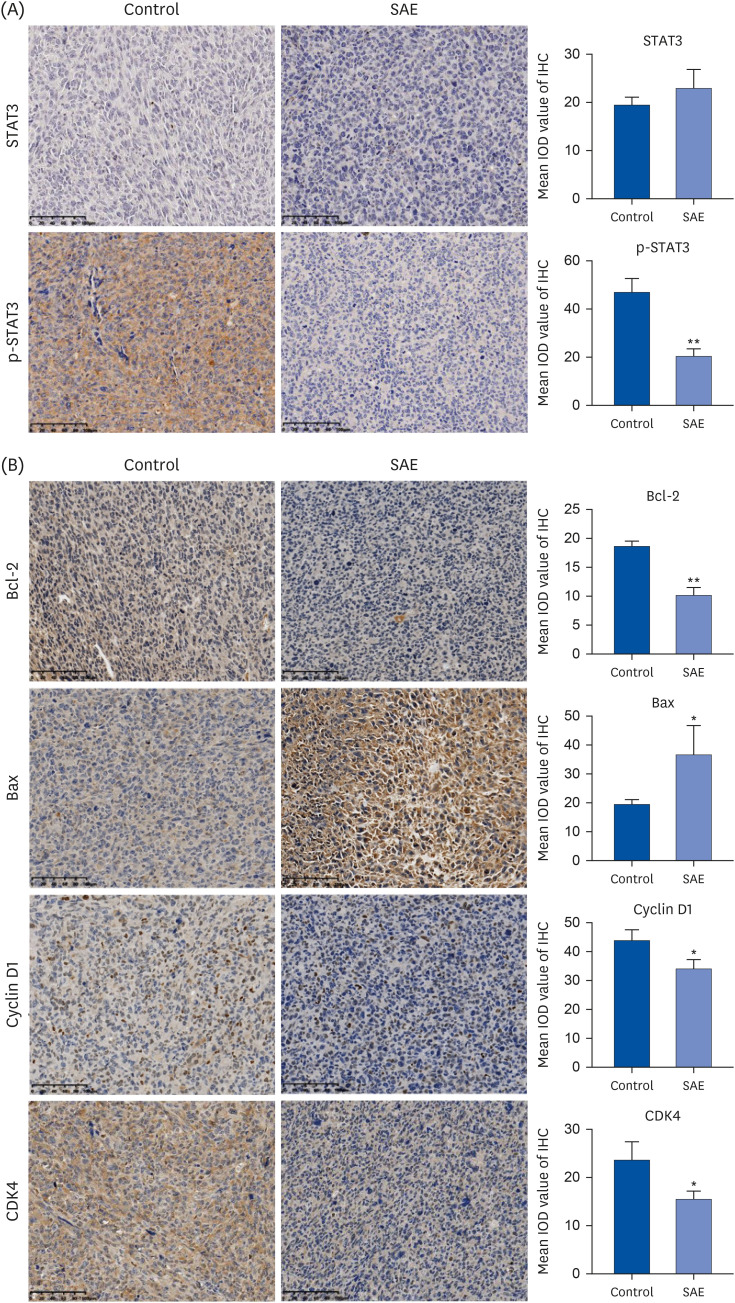
Different concentrations of SAE were used to culture lung cancer cells (A549 and H1650). A cell counting kit-8 assay was used to detect the survival ability of A549 and H1650 cells. A scratch assay and transwell cell invasion assay were used to detect the migration rate and invasive ability of SAE. Western blot analysis was used to detect the expression of B-cell lymphoma-2 (Bcl-2), Bcl2-associated X (Bax), cyclin D1, cyclin-dependent kinases 4 (CDK4), signal transducer and activator of transcription 3 (STAT3), and phosphorylated STAT3 (p-STAT3). Lung cancer xenograft mice were used to detect the inhibiting ability of SAE in vivo. Hematoxylin and eosin staining and immunohistochemistry were used to detect the effect of SAE on the structural changes to the tumor and the expression of Bcl-2, Bax, cyclin D1, CDK4, STAT3, and p-STAT3 in lung cancer xenograft mice.
RESULTS
SAE could inhibit lung cancer proliferation significantly in vitro and in vivo without cytotoxicity. SAE suppressed the viability, migration, and invasion of lung cancer cells in a dose and time-dependent manner. The SAE treatment significantly decreased the proapoptotic Bcl-2/Bax ratio and the expression of pro-proliferative proteins Cyclin D1 and CDK4 in vitro and in vivo. Furthermore, SAE also inhibited STAT3 expression.
CONCLUSIONS
SAE reduced the cell viability and suppressed cell migration and invasion in human lung cancer cells. Moreover, SAE also exhibited anti-proliferation effects in vivo. Therefore, SAE may have benefits in cancer therapy.
Keyword
Figure
Reference
-
1. Chen G, Zhang PG, Li JS, Duan JJ, Su W, Guo SP, Wang YF, Sun JN, Yang XT. Th17 cell frequency and IL-17A production in peripheral blood of patients with non-small-cell lung cancer. J Int Med Res. 2020; 48:300060520925948. PMID: 32600079.2. Wang L, Wang W. Safety and efficacy of anaplastic lymphoma kinase tyrosine kinase inhibitors in non-small cell lung cancer (review). [Review]. Oncol Rep. 2021; 45:13–28.3. Ding Y, Lu Y, Xie X, Sheng B, Wang Z. Silencing TRIM37 inhibits the proliferation and migration of non-small cell lung cancer cells. RSC Advances. 2018; 8:36852–36857. PMID: 35558931.4. Wang Y, Ding Y, Zhao J, Wang C, Gao M, Chi X, Zhang B, Ma X, Li L. Dihydroartemisinin and doxorubicin co-loaded Soluplus®-TPGS mixed micelles: formulation characterization, cellular uptake, and pharmacodynamic studies. Pharm Dev Technol. 2019; 24:1125–1132. PMID: 31305197.5. Bukowski K, Kciuk M, Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci. 2020; 21:3233–3257. PMID: 32370233.6. Hsiao WL, Liu L. The role of traditional Chinese herbal medicines in cancer therapy--from TCM theory to mechanistic insights. Planta Med. 2010; 76:1118–1131. PMID: 20635308.7. Dong J, Su SY, Wang MY, Zhan Z. Shenqi fuzheng, an injection concocted from Chinese medicinal herbs, combined with platinum-based chemotherapy for advanced non-small cell lung cancer: a systematic review. J Exp Clin Cancer Res. 2010; 29:137–148. PMID: 20969765.8. Cai C, Ma J, Han C, Jin Y, Zhao G, He X. Extraction and antioxidant activity of total triterpenoids in the mycelium of a medicinal fungus, Sanghuangporus sanghuang. Sci Rep. 2019; 9:7418–7428. PMID: 31092852.9. Konno S, Chu K, Feuer N, Phillips J, Choudhury M. Potent anticancer effects of bioactive mushroom extracts (Phellinus linteus) on a variety of human cancer cells. J Clin Med Res. 2015; 7:76–82. PMID: 25436023.10. Li G, Kim DH, Kim TD, Park BJ, Park HD, Park JI, Na MK, Kim HC, Hong ND, Lim K, et al. Protein-bound polysaccharide from Phellinus linteus induces G2/M phase arrest and apoptosis in SW480 human colon cancer cells. Cancer Lett. 2004; 216:175–181. PMID: 15533593.11. Zhu T, Guo J, Collins L, Kelly J, Xiao ZJ, Kim SH, Chen CY. Phellinus linteus activates different pathways to induce apoptosis in prostate cancer cells. Br J Cancer. 2007; 96:583–590. PMID: 17262078.12. Sliva D, Jedinak A, Kawasaki J, Harvey K, Slivova V. Phellinus linteus suppresses growth, angiogenesis and invasive behaviour of breast cancer cells through the inhibition of AKT signalling. Br J Cancer. 2008; 98:1348–1356. PMID: 18362935.13. Kiang KM, Leung GK. A review on adducin from functional to pathological mechanisms: future direction in cancer. BioMed Res Int. 2018; 2018:3465929. PMID: 29862265.14. Lin W, Zheng L, Zhuang Q, Zhao J, Cao Z, Zeng J, Lin S, Xu W, Peng J. Spica prunellae promotes cancer cell apoptosis, inhibits cell proliferation and tumor angiogenesis in a mouse model of colorectal cancer via suppression of STAT3 pathway. BMC Complement Altern Med. 2013; 13:144–154. PMID: 23800091.15. Chen Y, Wu H, Wang X, Wang C, Gan L, Zhu J, Tong J, Li Z. Huaier Granule extract inhibit the proliferation and metastasis of lung cancer cells through down-regulation of MTDH, JAK2/STAT3 and MAPK signaling pathways. Biomed Pharmacother. 2018; 101:311–321. PMID: 29499405.16. Yan JK, Wang YY, Ma HL, Wang ZB. Ultrasonic effects on the degradation kinetics, preliminary characterization and antioxidant activities of polysaccharides from Phellinus linteus mycelia. Ultrason Sonochem. 2016; 29:251–257. PMID: 26585005.17. Song KS, Li G, Kim JS, Jing K, Kim TD, Kim JP, Seo SB, Yoo JK, Park HD, Hwang BD, et al. Protein-bound polysaccharide from Phellinus linteus inhibits tumor growth, invasion, and angiogenesis and alters Wnt/β-catenin in SW480 human colon cancer cells. BMC Cancer. 2011; 11:307–318. PMID: 21781302.18. Li YG, Ji DF, Zhong S, Zhu JX, Chen S, Hu GY. Anti-tumor effects of proteoglycan from Phellinus linteus by immunomodulating and inhibiting Reg IV/EGFR/Akt signaling pathway in colorectal carcinoma. Int J Biol Macromol. 2011; 48:511–517. PMID: 21262260.19. Reheman D, Zhao J, Guan S, Xu GC, Li YJ, Sun SR. Apoptotic effect of novel pyrazolone-based derivative [Cu(PMPP-SAL)(EtOH)] on HeLa cells and its mechanism. Sci Rep. 2020; 10:18235–18247. PMID: 33106514.20. Ramesh P, Medema JP. BCL-2 family deregulation in colorectal cancer: potential for BH3 mimetics in therapy. Apoptosis. 2020; 25:305–320. PMID: 32335811.21. Cianfrocca R, Muscolini M, Marzano V, Annibaldi A, Marinari B, Levrero M, Costanzo A, Tuosto L. RelA/NF-kappaB recruitment on the bax gene promoter antagonizes p73-dependent apoptosis in costimulated T cells. Cell Death Differ. 2008; 15:354–363. PMID: 18034190.22. Bergandi L, Mungo E, Morone R, Bosco O, Rolando B, Doublier S. Hyperglycemia promotes chemoresistance through the reduction of the mitochondrial DNA damage, the Bax/Bcl-2 and Bax/Bcl-XL ratio, and the cells in sub-G1 phase due to antitumoral drugs induced-cytotoxicity in human colon adenocarcinoma cells. Front Pharmacol. 2018; 9:866–889. PMID: 30150934.23. Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008; 9:47–59. PMID: 18097445.24. Dong Y, Sui L, Sugimoto K, Tai Y, Tokuda M. Cyclin D1-CDK4 complex, a possible critical factor for cell proliferation and prognosis in laryngeal squamous cell carcinomas. Int J Cancer. 2001; 95:209–215. PMID: 11400112.25. Testa U, Pelosi E, Castelli G. Colorectal cancer: genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med Sci (Basel). 2018; 6:31–144. PMID: 29652830.26. Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003; 3:362–374. PMID: 12724734.27. Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014; 14:736–746. PMID: 25342631.28. Chai EZ, Shanmugam MK, Arfuso F, Dharmarajan A, Wang C, Kumar AP, Samy RP, Lim LH, Wang L, Goh BC, et al. Targeting transcription factor STAT3 for cancer prevention and therapy. Pharmacol Ther. 2016; 162:86–97. PMID: 26478441.29. Li Y, Wang Y, Shi Z, Liu J, Zheng S, Yang J, Liu Y, Yang Y, Chang F, Yu W. Clinicopathological and prognostic role of STAT3/p-STAT3 in breast cancer patients in China: a meta-analysis. Sci Rep. 2019; 9:11243–11252. PMID: 31375715.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transcriptome Analysis Reveals the Putative Polyketide Synthase Gene Involved in Hispidin Biosynthesis in Sanghuangporus sanghuang
- Inhibitory Effects of Toxoplasma Antigen on Proliferation and Invasion of Human Glioma Cells
- TIMP-2 gene transfer via adenovirus inhibits the invasion of lung cancer cell
- Circ_0001686 Promotes Prostate Cancer Progression by Up-Regulating SMAD3/TGFBR2 via miR-411-5p
- The MicroRNA hsa-let-7g Promotes Proliferation and Inhibits Apoptosis in Lung Cancer by Targeting HOXB1