Tuberc Respir Dis.
2013 Jul;75(1):9-17.
Autophagy Inhibition with Monensin Enhances Cell Cycle Arrest and Apoptosis Induced by mTOR or Epidermal Growth Factor Receptor Inhibitors in Lung Cancer Cells
- Affiliations
-
- 1Division of Pulmonology, Department of Internal Medicine, Korea Cancer Center Hospital, Seoul, Korea. cheol@kcch.re.kr
Abstract
- BACKGROUND
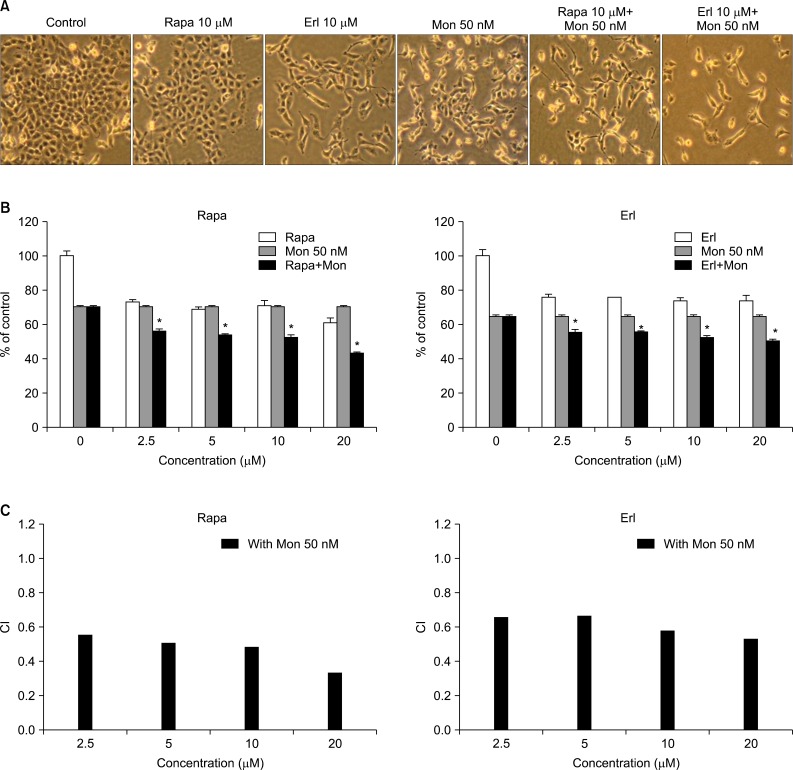
In cancer cells, autophagy is generally induced as a pro-survival mechanism in response to treatment-associated genotoxic and metabolic stress. Thus, concurrent autophagy inhibition can be expected to have a synergistic effect with chemotherapy on cancer cell death. Monensin, a polyether antibiotic, is known as an autophagy inhibitor, which interferes with the fusion of autophagosome and lysosome. There have been a few reports of its effect in combination with anticancer drugs. We performed this study to investigate whether erlotinib, an epidermal growth factor receptor inhibitor, or rapamycin, an mammalian target of rapamycin (mTOR) inhibitor, is effective in combination therapy with monensin in non-small cell lung cancer cells.
METHODS
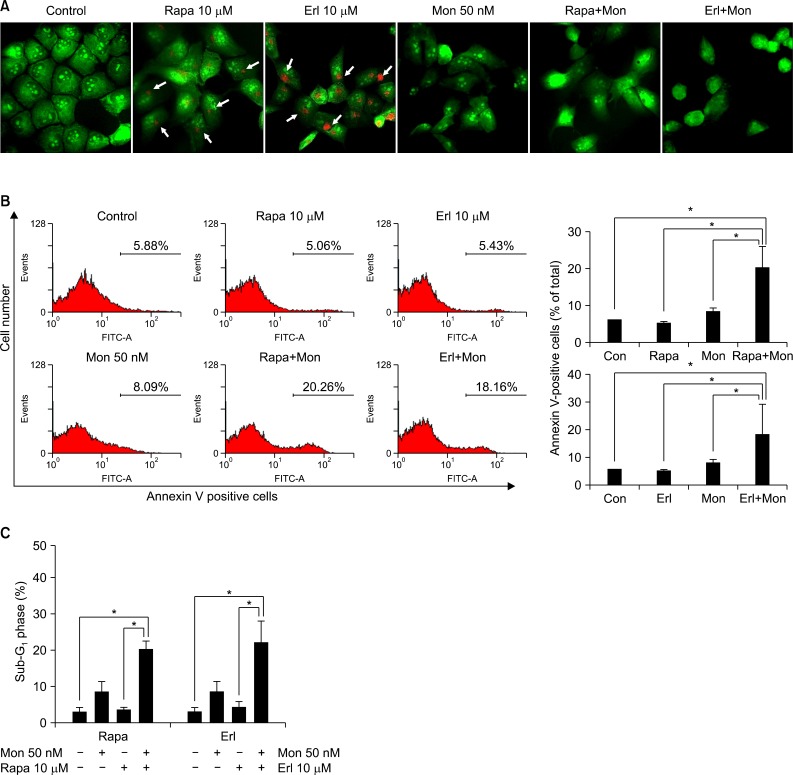
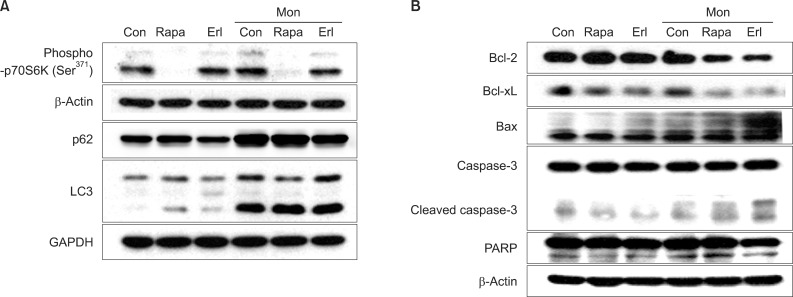
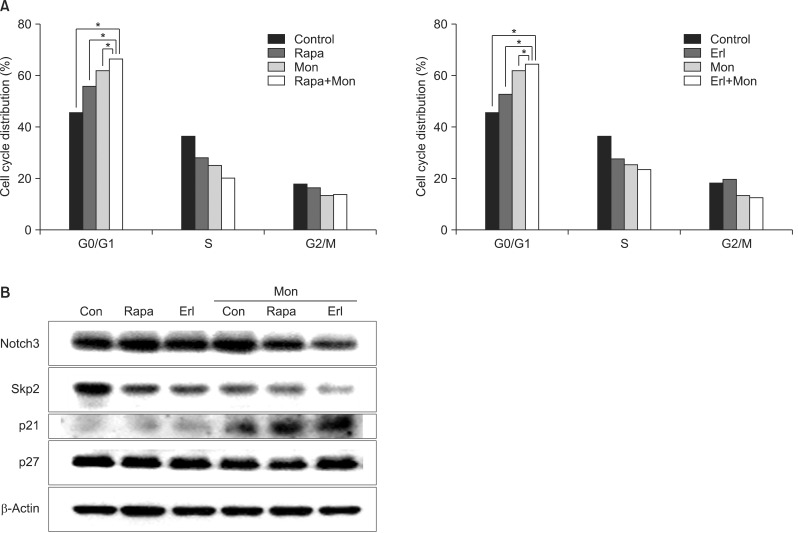
NCI-H1299 cells were treated with rapamycin or erlotinib, with or without monensin pretreatment, and then subjected to growth inhibition assay, apoptosis analysis by flow cytometry, and cell cycle analysis on the basis of the DNA contents histogram. Finally, a Western blot analysis was done to examine the changes of proteins related to apoptosis and cell cycle control.
RESULTS
Monensin synergistically increases growth inhibition and apoptosis induced by rapamycin or erlotinib. The number of cells in the sub-G1 phase increases noticeably after the combination treatment. Increase of proapoptotic proteins, including bax, cleaved caspase 3, and cleaved poly(ADP-ribose) polymerase, and decrease of anti-apoptotic proteins, bcl-2 and bcl-xL, are augmented by the combination treatment with monensin. The promoters of cell cycle progression, notch3 and skp2, decrease and p21, a cyclin-dependent kinase inhibitor, accumulates within the cell during this process.
CONCLUSION
Our findings suggest that concurrent autophagy inhibition could have a role in lung cancer treatment.
Keyword
MeSH Terms
-
Apoptosis
Apoptosis Regulatory Proteins
Autophagy
Blotting, Western
Carcinoma, Non-Small-Cell Lung
Caspase 3
Cell Cycle
Cell Cycle Checkpoints
Cell Death
DNA
Epidermal Growth Factor
Flow Cytometry
Lung
Lung Neoplasms
Lysosomes
Monensin
Phosphotransferases
Poly(ADP-ribose) Polymerases
Proteins
Quinazolines
Receptor, Epidermal Growth Factor
Receptor, erbB-2
Sirolimus
Stress, Physiological
TOR Serine-Threonine Kinases
Erlotinib Hydrochloride
Apoptosis Regulatory Proteins
Caspase 3
DNA
Epidermal Growth Factor
Monensin
Phosphotransferases
Poly(ADP-ribose) Polymerases
Proteins
Quinazolines
Receptor, Epidermal Growth Factor
Receptor, erbB-2
Sirolimus
TOR Serine-Threonine Kinases
Figure
Reference
-
1. Marx J. Autophagy: is it cancer's friend or foe? Science. 2006; 312:1160–1161. PMID: 16728626.
Article2. Hippert MM, O'Toole PS, Thorburn A. Autophagy in cancer: good, bad, or both? Cancer Res. 2006; 66:9349–9351. PMID: 17018585.3. Turcotte S, Giaccia AJ. Targeting cancer cells through autophagy for anticancer therapy. Curr Opin Cell Biol. 2010; 22:246–251. PMID: 20056398.
Article4. Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005; 5:726–734. PMID: 16148885.
Article5. Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005; 25:1025–1040. PMID: 15657430.
Article6. Huczynski A, Stefanska J, Przybylski P, Brzezinski B, Bartl F. Synthesis and antimicrobial properties of monensin A esters. Bioorg Med Chem Lett. 2008; 18:2585–2589. PMID: 18375122.7. Hirsch FR, Scagliotti GV, Langer CJ, Varella-Garcia M, Franklin WA. Epidermal growth factor family of receptors in preneoplasia and lung cancer: perspectives for targeted therapies. Lung Cancer. 2003; 41(Suppl 1):S29–S42. PMID: 12867060.
Article8. Scagliotti GV, Selvaggi G, Novello S, Hirsch FR. The biology of epidermal growth factor receptor in lung cancer. Clin Cancer Res. 2004; 10(12 Pt 2):4227s–4232s. PMID: 15217963.
Article9. Han W, Pan H, Chen Y, Sun J, Wang Y, Li J, et al. EGFR tyrosine kinase inhibitors activate autophagy as a cytoprotective response in human lung cancer cells. PLoS One. 2011; 6:e18691. PMID: 21655094.
Article10. Takezawa K, Okamoto I, Tanizaki J, Kuwata K, Yamaguchi H, Fukuoka M, et al. Enhanced anticancer effect of the combination of BIBW2992 and thymidylate synthase-targeted agents in non-small cell lung cancer with the T790M mutation of epidermal growth factor receptor. Mol Cancer Ther. 2010; 9:1647–1656. PMID: 20530710.
Article11. Sos ML, Rode HB, Heynck S, Peifer M, Fischer F, Kluter S, et al. Chemogenomic profiling provides insights into the limited activity of irreversible EGFR Inhibitors in tumor cells expressing the T790M EGFR resistance mutation. Cancer Res. 2010; 70:868–874. PMID: 20103621.12. Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009; 27:2278–2287. PMID: 19332717.
Article13. Zou ZQ, Zhang XH, Wang F, Shen QJ, Xu J, Zhang LN, et al. A novel dual PI3Kalpha/mTOR inhibitor PI-103 with high antitumor activity in non-small cell lung cancer cells. Int J Mol Med. 2009; 24:97–101. PMID: 19513541.
Article14. Sheppard K, Kinross KM, Solomon B, Pearson RB, Phillips WA. Targeting PI3 kinase/AKT/mTOR signaling in cancer. Crit Rev Oncog. 2012; 17:69–95. PMID: 22471665.
Article15. Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007; 7:961–967. PMID: 17972889.
Article16. Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006; 10:51–64. PMID: 16843265.
Article17. Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007; 117:326–336. PMID: 17235397.
Article18. Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011; 17:654–666. PMID: 21325294.
Article19. Svensson JP, Fry RC, Wang E, Somoza LA, Samson LD. Identification of novel human damage response proteins targeted through yeast orthology. PLoS One. 2012; 7:e37368. PMID: 22615993.
Article20. Hou W, Han J, Lu C, Goldstein LA, Rabinowich H. Autophagic degradation of active caspase-8: a crosstalk mechanism between autophagy and apoptosis. Autophagy. 2010; 6:891–900. PMID: 20724831.21. Li X, Lu Y, Pan T, Fan Z. Roles of autophagy in cetuximab-mediated cancer therapy against EGFR. Autophagy. 2010; 6:1066–1077. PMID: 20864811.
Article22. Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, et al. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem. 2010; 285:10850–10861. PMID: 20123989.
Article23. Powis G, Bonjouklian R, Berggren MM, Gallegos A, Abraham R, Ashendel C, et al. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994; 54:2419–2423. PMID: 8162590.24. Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelarova H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem. 1997; 243:240–246. PMID: 9030745.
Article25. Kaini RR, Hu CA. Synergistic killing effect of chloroquine and androgen deprivation in LNCaP cells. Biochem Biophys Res Commun. 2012; 425:150–156. PMID: 22819840.
Article26. Rahim R, Strobl JS. Hydroxychloroquine, chloroquine, and all-trans retinoic acid regulate growth, survival, and histone acetylation in breast cancer cells. Anticancer Drugs. 2009; 20:736–745. PMID: 19584707.
Article27. Klionsky DJ, Elazar Z, Seglen PO, Rubinsztein DC. Does bafilomycin A1 block the fusion of autophagosomes with lysosomes? Autophagy. 2008; 4:849–950. PMID: 18758232.28. Swampillai AL, Salomoni P, Short SC. The role of autophagy in clinical practice. Clin Oncol (R Coll Radiol). 2012; 24:387–395. PMID: 22032864.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antiproliferative Activity of Gibbosic Acid H through Induction of G0/G1 Cell Cycle Arrest and Apoptosis in Human Lung Cancer Cells
- Apoptosis and Cell Cycle Arrest with EGF, TGF- a and TGF- 8 in Cervical Cancer Cell Lines
- Inhibition of Ubiquitin-specific Peptidase 8 Suppresses Growth of Gefitinib-resistant Non-small Cell Lung Cancer Cells by Inducing Apoptosis
- Autophagy-Dependent Survival of Mutant B-Raf Melanoma Cells Selected for Resistance to Apoptosis Induced by Inhibitors against Oncogenic B-Raf
- Dasatinib induces apoptosis and autophagy by suppressing the PI3K/Akt/mTOR pathway in bladder cancer cells