J Menopausal Med.
2015 Aug;21(2):93-103. 10.6118/jmm.2015.21.2.93.
Effect of Glucagon-like Peptide-1 on the Differentiation of Adipose-derived Stem Cells into Osteoblasts and Adipocytes
- Affiliations
-
- 1Department of Molecular Biology, Natural Science College, Pusan National University, Busan, Korea.
- 2Research Center for Anti-Aging Technology Development, Pusan National University, Busan, Korea.
- 3Department of Obstetrics and Gynecology, Kosin University Hospital, Busan, Korea.
- 4Department of Obstetrics and Gynecology, Busan Adventist Hospital, Busan, Korea. hykyale@hanamil.net
- 5Department of Pediatric Cardiology, Dona-A University Hospital, Busan, Korea. lyspedia@dau.ac.kr
- KMID: 2200986
- DOI: http://doi.org/10.6118/jmm.2015.21.2.93
Abstract
OBJECTIVES
Glucagon-like peptide-1 (GLP-1) is an intestinally secreted hormone and it plays an important role in the regulation of glucose homeostasis. However, the possible role of GLP-1 in the differentiation of adipose-derived stem cells (ADSCs) remains unknown. Therefore this study investigated the effect of GLP-1 on the differentiation of ADSCs into osteoblasts and adipocytes.
METHODS
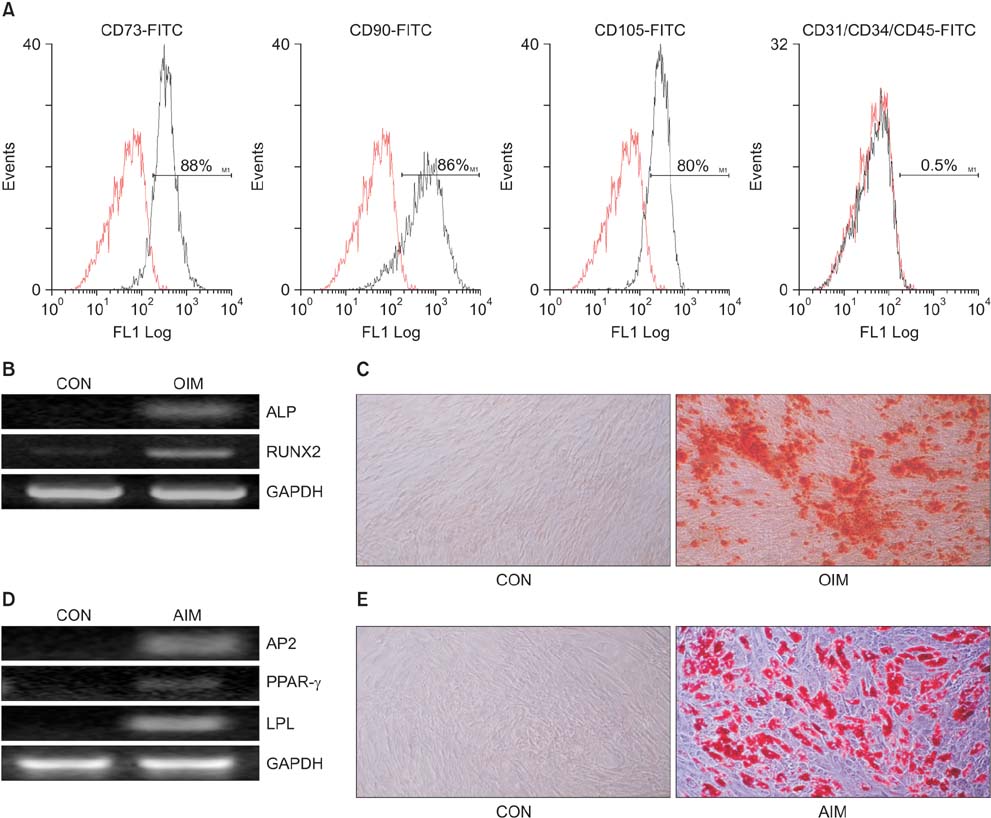
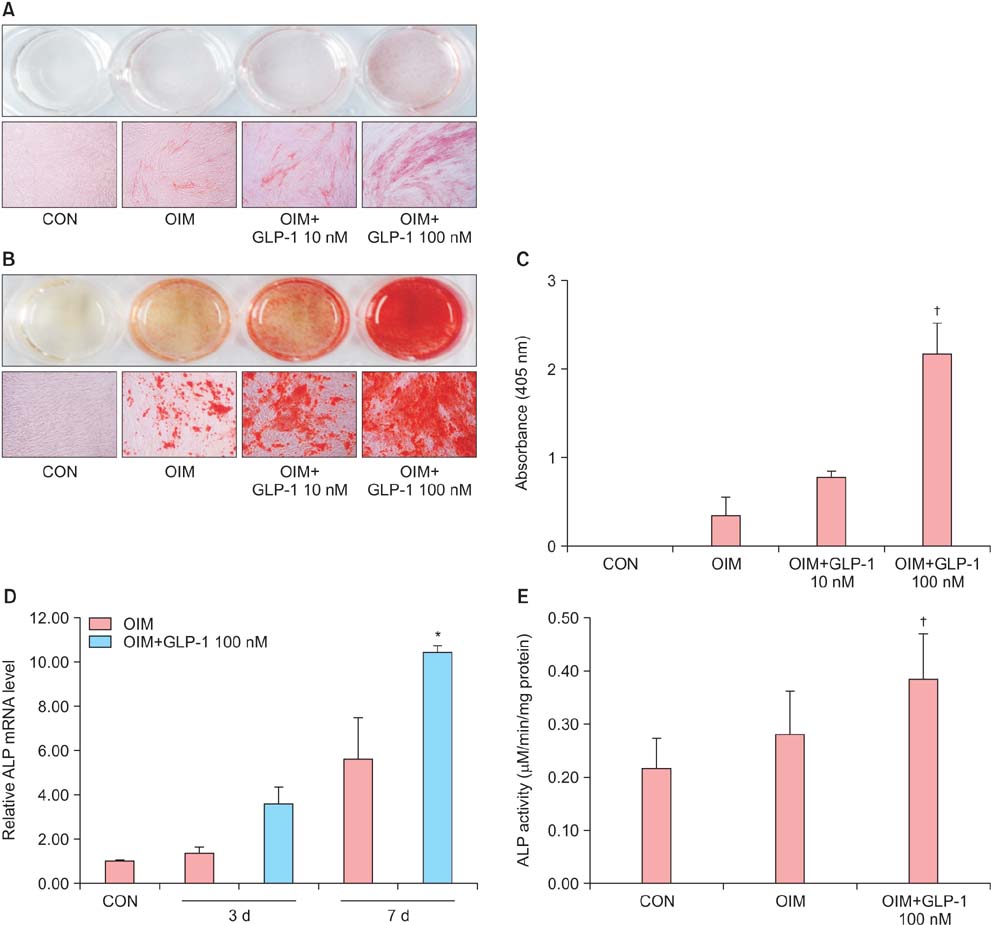
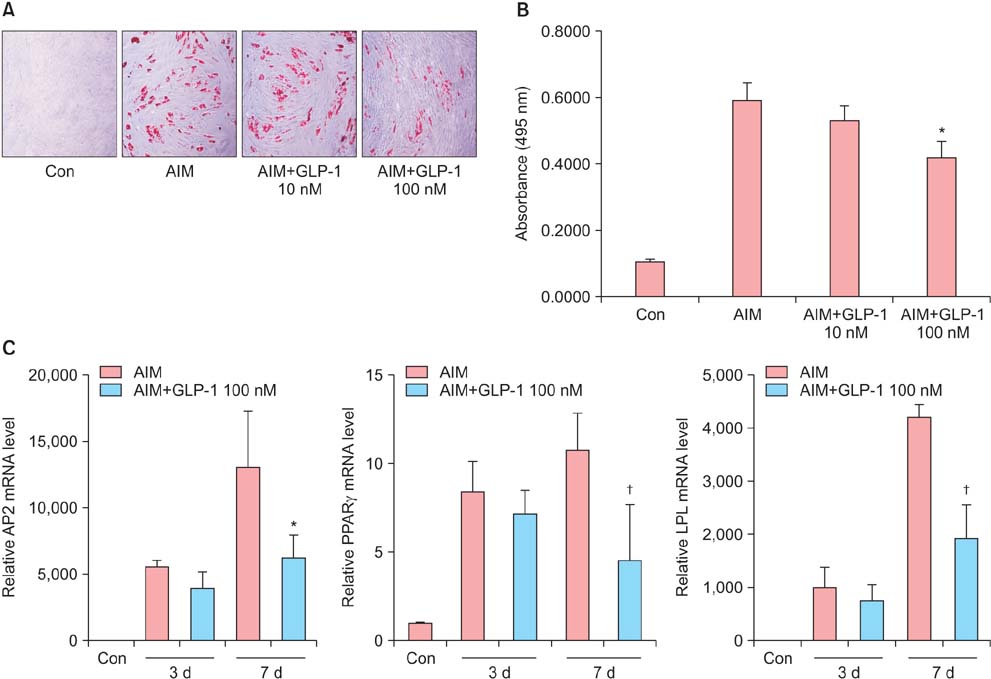
ADSCs were isolated from human adipose tissues of the abdomens, cultured and characterized by flow cytometry and multi-lineage potential assay. ADSCs were induced in osteogenic and adipogenic media treated with two different doses (10 and 100 nM) of GLP-1, and then the effect of GLP-1 on differentiation of ADSCs into osteoblast and adipocyte was examined. The signaling pathway involved in these processes was also examined.
RESULTS
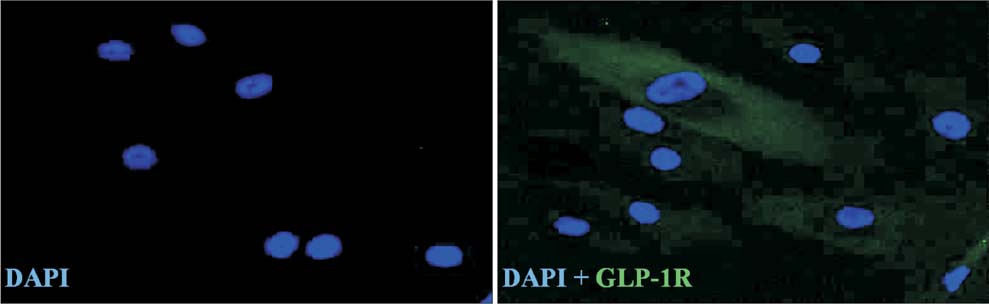
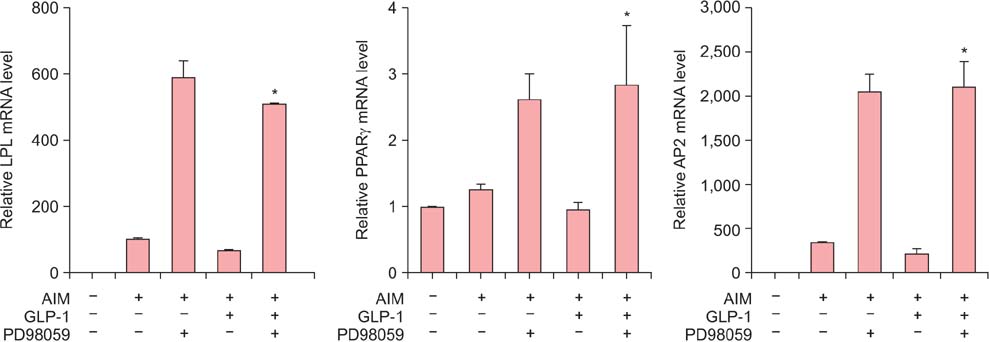
Isolated human ADSCs expressed mesenchymal stem cell (MSC) specific markers as well as GLP-1 receptor (GLP-1R) proteins. They also showed multiple-lineage potential of MSC. GLP-1 was upregulated the activity and mRNA expression of osteoblast-specific marker, alkaline phosphatase and the mineralization of calcium. In contrast, GLP-1 significantly suppressed the expression of adipocyte-specific markers, peroxisome proliferator-activated receptor gamma (PPAR-gamma), lipoprotein lipase (LPL) and adipocyte protein 2 (AP2). This decreased expression of adipocyte specific markers caused by GLP-1 was significantly reversed by the treatment of extracellular signal-regulated kinase (ERK) inhibitor, PD98059 (P < 0.05).
CONCLUSION
This result demonstrates that GLP-1 stimulates osteoblast differentiation in ADSCs, whereas it inhibits adipocyte differentiation. The ERK signaling pathway seems to be involved in these differentiation processes mediated by GLP-1.
Keyword
MeSH Terms
-
Abdomen
Adipocytes*
Adipogenesis
Adipose Tissue
Alkaline Phosphatase
Calcium
Cell Differentiation
Flow Cytometry
Glucagon-Like Peptide 1*
Glucose
Homeostasis
Humans
Lipoprotein Lipase
Mesenchymal Stromal Cells
Osteoblasts*
Osteogenesis
Phosphotransferases
PPAR gamma
RNA, Messenger
Stem Cells*
Glucagon-Like Peptide-1 Receptor
Alkaline Phosphatase
Calcium
Glucagon-Like Peptide 1
Glucose
Lipoprotein Lipase
PPAR gamma
Phosphotransferases
RNA, Messenger
Figure
Reference
-
1. Schwartz AV. TZDs and Bone: A Review of the Recent Clinical Evidence. PPAR Res. 2008; 2008:297893.2. Schwartz AV, Sellmeyer DE, Vittinghoff E, Palermo L, Lecka-Czernik B, Feingold KR, et al. Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab. 2006; 91:3349–3354.3. Duque G. Bone and fat connection in aging bone. Curr Opin Rheumatol. 2008; 20:429–434.4. Barnett A. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. Int J Clin Pract. 2006; 60:1454–1470.5. Creutzfeldt W. The entero-insular axis in type 2 diabetes--incretins as therapeutic agents. Exp Clin Endocrinol Diabetes. 2001; 109:Suppl 2. S288–S303.6. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007; 87:1409–1439.7. Ma X, Hui H, Liu Z, He G, Hu J, Meng J, et al. Poly-GLP-1, a novel long-lasting glucagon-like peptide-1 polymer, ameliorates hyperglycaemia by improving insulin sensitivity and increasing pancreatic beta-cell proliferation. Diabetes Obes Metab. 2009; 11:953–965.8. Alvarez E, Martinez MD, Roncero I, Chowen JA, Garcia-Cuartero B, Gispert JD, et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem. 2005; 92:798–806.9. Alvarez E, Roncero I, Chowen JA, Thorens B, Blazquez E. Expression of the glucagon-like peptide-1 receptor gene in rat brain. J Neurochem. 1996; 66:920–927.10. Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004; 109:962–965.11. Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992; 326:1316–1322.12. Toft-Nielsen MB, Madsbad S, Holst JJ. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab. 2001; 86:3853–3860.13. Singh S, Chang HY, Richards TM, Weiner JP, Clark JM, Segal JB. Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med. 2013; 173:534–539.14. Sanz C, Vazquez P, Blazquez C, Barrio PA, Alvarez Mdel M, Blazquez E. Signaling and biological effects of glucagon-like peptide 1 on the differentiation of mesenchymal stem cells from human bone marrow. Am J Physiol Endocrinol Metab. 2010; 298:E634–E643.15. Yamada C, Yamada Y, Tsukiyama K, Yamada K, Udagawa N, Takahashi N, et al. The murine glucagon-like peptide-1 receptor is essential for control of bone resorption. Endocrinology. 2008; 149:574–579.16. Nuche-Berenguer B, Lozano D, Gutierrez-Rojas I, Moreno P, Marinoso ML, Esbrit P, et al. GLP-1 and exendin-4 can reverse hyperlipidic-related osteopenia. J Endocrinol. 2011; 209:203–210.17. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147.18. Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004; 116:639–648.19. Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004; 22:560–567.20. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002; 13:4279–4295.21. Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992; 102(Pt 2):341–351.22. Hong JH, Yaffe MB. TAZ: a beta-catenin-like molecule that regulates mesenchymal stem cell differentiation. Cell Cycle. 2006; 5:176–179.23. Lee BI. Hormone replacement therapy following stem cell transplant. J Korean Soc Menopause. 2007; 13:8–13.24. Nuche-Berenguer B, Moreno P, Esbrit P, Dapia S, Caeiro JR, Cancelas J, et al. Effect of GLP-1 treatment on bone turnover in normal, type 2 diabetic, and insulin-resistant states. Calcif Tissue Int. 2009; 84:453–461.25. Quoyer J, Longuet C, Broca C, Linck N, Costes S, Varin E, et al. GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a beta-arrestin 1-mediated ERK1/2 activation in pancreatic beta-cells. J Biol Chem. 2010; 285:1989–2002.26. Nuche-Berenguer B, Portal-Nunez S, Moreno P, Gonzalez N, Acitores A, Lopez-Herradon A, et al. Presence of a functional receptor for GLP-1 in osteoblastic cells, independent of the cAMP-linked GLP-1 receptor. J Cell Physiol. 2010; 225:585–592.27. Pacheco-Pantoja EL, Ranganath LR, Gallagher JA, Wilson PJ, Fraser WD. Receptors and effects of gut hormones in three osteoblastic cell lines. BMC Physiol. 2011; 11:12.28. Challa TD, Beaton N, Arnold M, Rudofsky G, Langhans W, Wolfrum C. Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J Biol Chem. 2012; 287:6421–6430.29. Aubin JE, Liu F, Malaval L, Gupta AK. Osteoblast and chondroblast differentiation. Bone. 1995; 17:77S–83S.30. Golub EE, Boesze-Battaglia K. The role of alkaline phosphatase in mineralization. Curr Opin Orthop. 2007; 18:444–448.31. Ahn KH, Jung SE, Yi KW, Park HT, Shin JH, Kim YT, et al. Barium stimulates the expression of osteogenic genes in human mesenchymal stem cells into osteoblasts in vitro. J Korean Soc Menopause. 2011; 17:81–87.32. Yang J, Ren J, Song J, Liu F, Wu C, Wang X, et al. Glucagon-like peptide 1 regulates adipogenesis in 3T3-L1 preadipocytes. Int J Mol Med. 2013; 31:1429–1435.33. Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971; 80:147–154.34. Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004; 3:379–389.35. Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004; 113:846–855.36. Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001; 2:165–171.37. Choi SS, Cha BY, Iida K, Sato M, Lee YS, Teruya T, et al. Honokiol enhances adipocyte differentiation by potentiating insulin signaling in 3T3-L1 preadipocytes. J Nat Med. 2011; 65:424–430.38. Zhang M, Ikeda K, Xu JW, Yamori Y, Gao XM, Zhang BL. Genistein suppresses adipogenesis of 3T3-L1 cells via multiple signal pathways. Phytother Res. 2009; 23:713–718.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adipose-derived stem cells: characterization and clinical application
- Expression of Glucagon-Like Peptide 1 Receptor during Osteogenic Differentiation of Adipose-Derived Stem Cells
- A study on the osteogenic differentiation of adipose-derived adult stem cell
- Histological Analysis of In Vitro Co-Culture and In Vivo Mice Co-Transplantation of Stem Cell-Derived Adipocyte and Osteoblast
- Differentiation Potential of Mesenchymal Stem Cells Is Related to Their Intrinsic Mechanical Properties