Endocrinol Metab.
2014 Dec;29(4):567-573. 10.3803/EnM.2014.29.4.567.
Expression of Glucagon-Like Peptide 1 Receptor during Osteogenic Differentiation of Adipose-Derived Stem Cells
- Affiliations
-
- 1Department of Internal Medicine, Pusan National University School of Medicine, Korea. injkim@pusan.ac.kr
- 2Biomedical Research Institute, Pusan National University, Korea.
- 3Kim Yong Ki Internal Medicine Clinic, Korea.
- 4Department of Plastic and Reconstructive Surgery, Pusan National University Hospital, Korea.
- 5Division of Endocrinology and Metabolism, Department of Internal Medicine, Kosin University College of Medicine, Korea.
- 6Department of Internal Medicine, Good Moonhwa Hospital, Busan, Korea.
- KMID: 2384256
- DOI: http://doi.org/10.3803/EnM.2014.29.4.567
Abstract
- BACKGROUND
Glucagon-like peptide 1 (GLP-1), an incretin hormone well known for its glucose-lowering effect, was recently reported to exert an anabolic effect on bone. Although the exact mechanism is not known, it likely involves the GLP-1 receptor (GLP-1R), which is expressed in some osteoblastic cell lines. Adipose-derived stem cells (ADSCs) have mesenchymal stem cell-specific characteristics, including osteoblastic differentiation potential. We evaluated the expression of GLP-1R during osteogenic differentiation of ADSCs.
METHODS
ADSCs were isolated from subcutaneous adipose tissue obtained from three male donors during plastic surgery and were subjected to osteogenic induction. Mineralization was assessed by Alizarin Red staining on day 21. Expression of alkaline phosphatase (ALP), osteocalcin (OC), and GLP-1R was measured by real-time polymerase chain reaction in triplicate for each patient on days 0, 7, 14, and 21. Target mRNA expression levels were normalized to that of beta-actin.
RESULTS
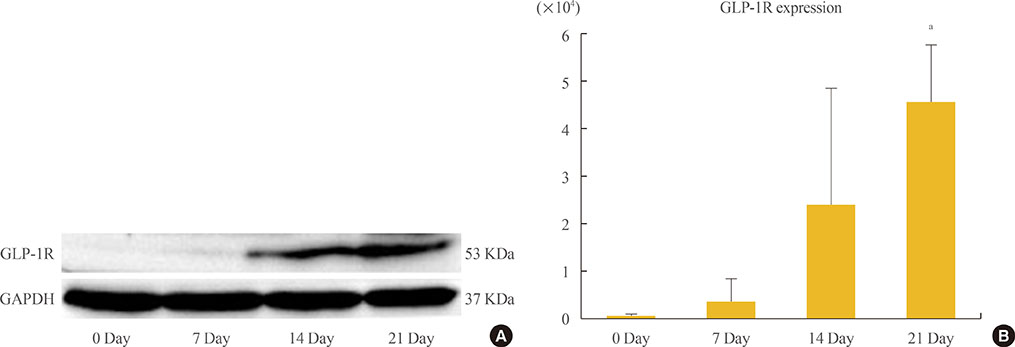
ADSCs were fibroblast-like in morphology, adhered to plastic, and had multipotent differentiation potential, as assessed using specific antigen markers. The osteogenic markers ALP and OC were notably upregulated at 21 days. Osteogenic differentiation resulted in a time-dependent increase in the expression of GLP-1R (P=0.013).
CONCLUSION
We demonstrated upregulation of GLP-1R gene expression during osteogenic differentiation of ADSCs. This finding suggests that GLP-1 may induce osteogenic differentiation in bone tissue.
Keyword
MeSH Terms
-
Actins
Alkaline Phosphatase
Anabolic Agents
Bone and Bones
Cell Line
Gene Expression
Glucagon-Like Peptide 1*
Humans
Incretins
Male
Osteoblasts
Osteocalcin
Osteogenesis
Real-Time Polymerase Chain Reaction
RNA, Messenger
Stem Cells*
Subcutaneous Fat
Surgery, Plastic
Tissue Donors
Up-Regulation
Glucagon-Like Peptide-1 Receptor
Actins
Alkaline Phosphatase
Anabolic Agents
Glucagon-Like Peptide 1
Incretins
Osteocalcin
RNA, Messenger
Figure
Reference
-
1. Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol. 2003; 17:161–171.2. Nuche-Berenguer B, Lozano D, Gutierrez-Rojas I, Moreno P, Marinoso ML, Esbrit P, Villanueva-Penacarrillo ML. GLP-1 and exendin-4 can reverse hyperlipidic-related osteopenia. J Endocrinol. 2011; 209:203–210.3. Nuche-Berenguer B, Moreno P, Esbrit P, Dapia S, Caeiro JR, Cancelas J, Haro-Mora JJ, Villanueva-Penacarrillo ML. Effect of GLP-1 treatment on bone turnover in normal, type 2 diabetic, and insulin-resistant states. Calcif Tissue Int. 2009; 84:453–461.4. Nuche-Berenguer B, Portal-Nunez S, Moreno P, Gonzalez N, Acitores A, Lopez-Herradon A, Esbrit P, Valverde I, Villanueva-Penacarrillo ML. Presence of a functional receptor for GLP-1 in osteoblastic cells, independent of the cAMP-linked GLP-1 receptor. J Cell Physiol. 2010; 225:585–592.5. Pacheco-Pantoja EL, Ranganath LR, Gallagher JA, Wilson PJ, Fraser WD. Receptors and effects of gut hormones in three osteoblastic cell lines. BMC Physiol. 2011; 11:12.6. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002; 13:4279–4295.7. Rodriguez AM, Elabd C, Amri EZ, Ailhaud G, Dani C. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005; 87:125–128.8. Gimble JM, Guilak F. Differentiation potential of adipose derived adult stem (ADAS) cells. Curr Top Dev Biol. 2003; 58:137–160.9. Witkowska-Zimny M, Walenko K. Stem cells from adipose tissue. Cell Mol Biol Lett. 2011; 16:236–257.10. Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006; 99:1285–1297.11. Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004; 22:560–567.12. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317.13. Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006; 8:436–447.14. Ma X, Meng J, Jia M, Bi L, Zhou Y, Wang Y, Hu J, He G, Luo X. Exendin-4, a glucagon-like peptide-1 receptor agonist, prevents osteopenia by promoting bone formation and suppressing bone resorption in aged ovariectomized rats. J Bone Miner Res. 2013; 28:1641–1652.15. Kim JY, Lee SK, Jo KJ, Song DY, Lim DM, Park KY, Bonewald LF, Kim BJ. Exendin-4 increases bone mineral density in type 2 diabetic OLETF rats potentially through the down-regulation of SOST/sclerostin in osteocytes. Life Sci. 2013; 92:533–540.16. Yamada C, Yamada Y, Tsukiyama K, Yamada K, Udagawa N, Takahashi N, Tanaka K, Drucker DJ, Seino Y, Inagaki N. The murine glucagon-like peptide-1 receptor is essential for control of bone resorption. Endocrinology. 2008; 149:574–579.17. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007; 87:1409–1439.18. Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL, Hegelund AC, Jacobsen H, Jacobsen SD, Moses AC, Molck AM, Nielsen HS, Nowak J, Solberg H, Thi TD, Zdravkovic M, Moerch U. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010; 151:1473–1486.19. Hirsch PF, Baruch H. Is calcitonin an important physiological substance? Endocrine. 2003; 21:201–208.20. Boyan BD, Schwartz Z, Boskey AL. The importance of mineral in bone and mineral research. Bone. 2000; 27:341–342.21. Declercq HA, Verbeeck RM, De Ridder LI, Schacht EH, Cornelissen MJ. Calcification as an indicator of osteoinductive capacity of biomaterials in osteoblastic cell cultures. Biomaterials. 2005; 26:4964–4974.22. Aubin JE, Liu F, Malaval L, Gupta AK. Osteoblast and chondroblast differentiation. Bone. 1995; 17:2 Suppl. 77S–83S.23. Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol. 1990; 143:213–221.24. Koshihara Y, Kawamura M, Endo S, Tsutsumi C, Kodama H, Oda H, Higaki S. Establishment of human osteoblastic cells derived from periosteum in culture. In Vitro Cell Dev Biol. 1989; 25:37–43.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Glucagon-like Peptide-1 on the Differentiation of Adipose-derived Stem Cells into Osteoblasts and Adipocytes
- Osteogenic Differentiation of Human Adipose-derived Stem Cells within PLGA(Poly(D,L-lactic-co-glycolic acid)) Scaffold in the Nude Mouse
- A study on the osteogenic differentiation of adipose-derived adult stem cell
- Letter: Expression of Glucagon-Like Peptide-1 Receptor in Papillary Thyroid Carcinoma and Its Clinicopathologic Significance (Endocrinol Metab 2014;29:536-44, Min Jung Jung et al.)
- Modulation of Osteogenic Differentiation of Adipose-Derived Stromal Cells by Co-Treatment with 3, 4’-Dihydroxyflavone, U0126, and N-Acetyl Cysteine