Korean J Physiol Pharmacol.
2020 Jan;24(1):81-88. 10.4196/kjpp.2020.24.1.81.
Spatiotemporal expression of RCAN1 and its isoform RCAN1-4 in the mouse hippocampus after pilocarpine-induced status epilepticus
- Affiliations
-
- 1Department of Pharmacology, Department of Biomedicine & Health Sciences, Catholic Neuroscience Institute, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea. syk@catholic.ac.kr
- 2Institute of Aging and Metabolic Diseases, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- 3Department of Anatomy, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- KMID: 2466572
- DOI: http://doi.org/10.4196/kjpp.2020.24.1.81
Abstract
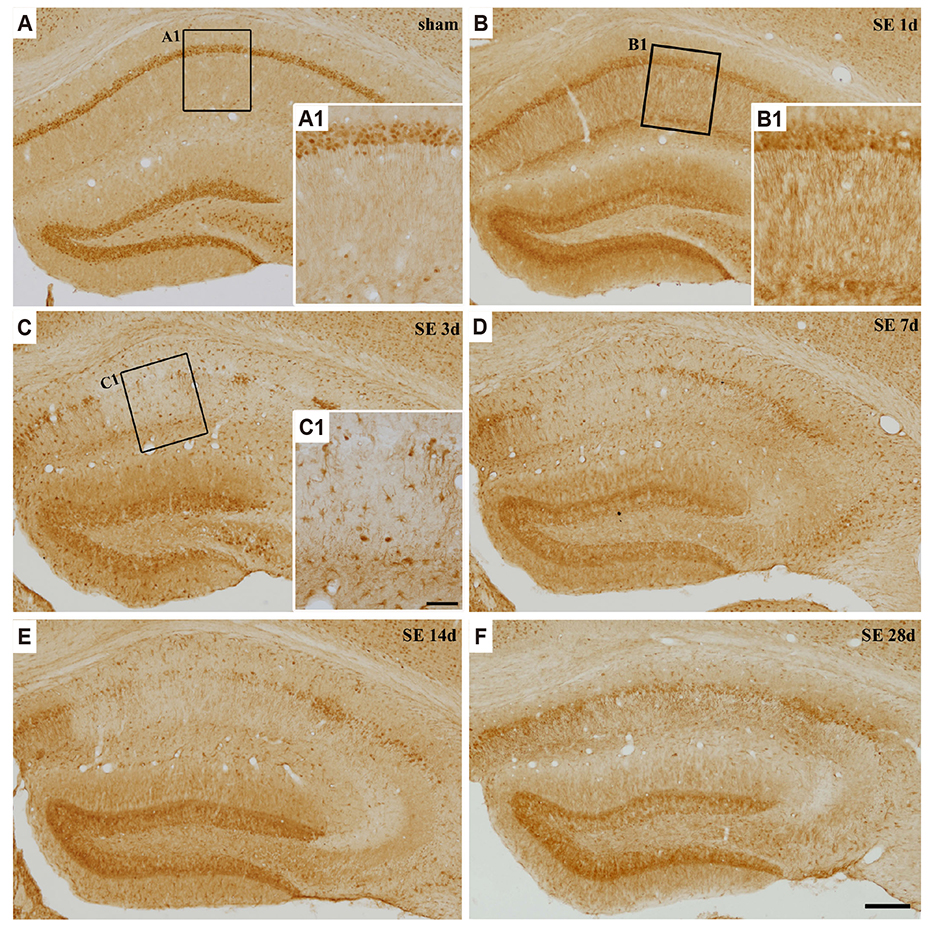
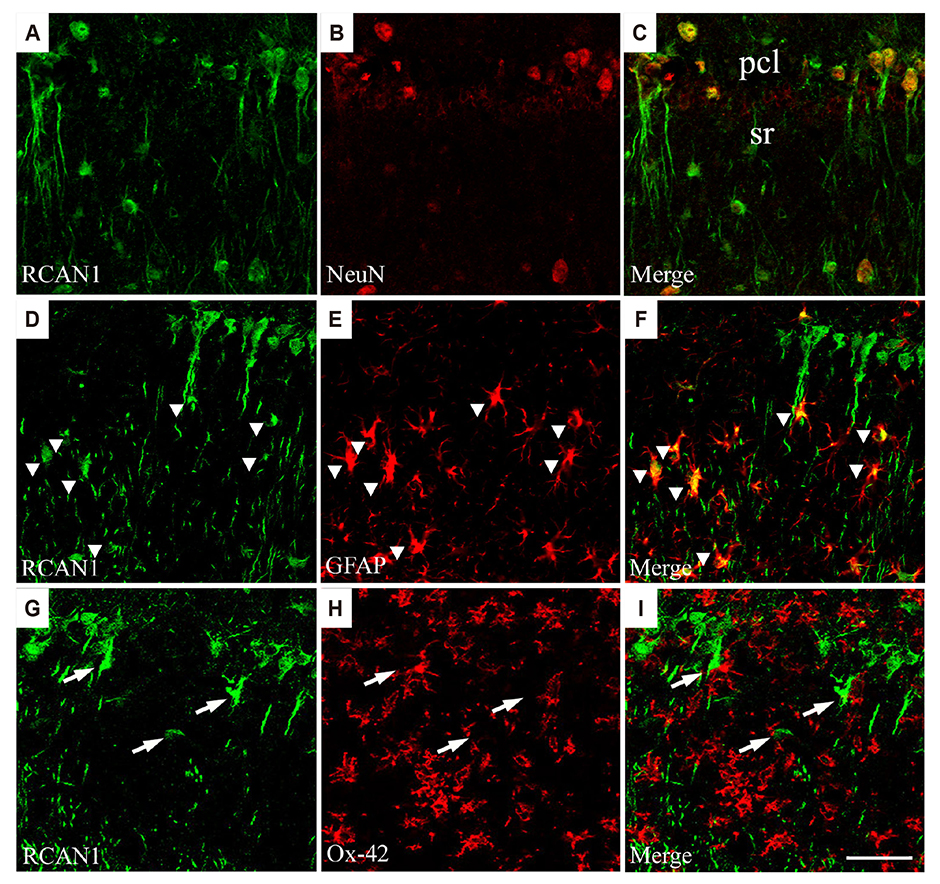
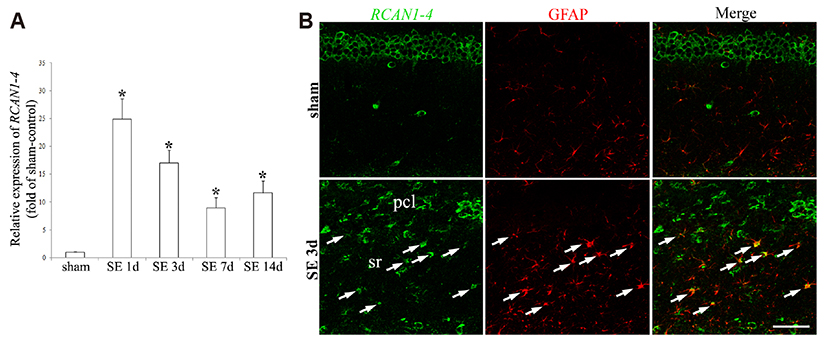
- Regulator of calcineurin 1 (RCAN1) can be induced by an intracellular calcium increase and oxidative stress, which are characteristic features of temporal lobe epilepsy. Thus, we investigated the spatiotemporal expression and cellular localization of RCAN1 protein and mRNA in the mouse hippocampus after pilocarpine-induced status epilepticus (SE). Male C57BL/6 mice were given pilocarpine hydrochloride (280 mg/kg, i.p.) and allowed to develop 2 h of SE. Then the animals were given diazepam (10 mg/kg, i.p.) to stop the seizures and sacrificed at 1, 3, 7, 14, or 28 day after SE. Cresyl violet staining showed that pilocarpine-induced SE resulted in cell death in the CA1 and CA3 subfields of the hippocampus from 3 day after SE. RCAN1 immunoreactivity showed that RCAN1 was mainly expressed in neurons in the shammanipulated hippocampi. At 1 day after SE, RCAN1 expression became detected in hippocampal neuropils. However, RCAN1 signals were markedly enhanced in cells with stellate morphology at 3 and 7 day after SE, which were confirmed to be reactive astrocytes, but not microglia by double immunofluorescence. In addition, real-time reverse transcriptase-polymerase chain reaction showed a significant upregulation of RCAN1 isoform 4 (RCAN1-4) mRNA in the SE-induced hippocampi. Finally, in situ hybridization with immunohistochemistry revealed astrocytic expression of RCAN1-4 after SE. These results demonstrate astrocytic upregulation of RCAN1 and RCAN1-4 in the mouse hippocampus in the acute and subacute phases of epileptogenesis, providing foundational information for the potential role of RCAN1 in reactive astrocytes during epileptogenesis.
Keyword
MeSH Terms
-
Animals
Astrocytes
Calcineurin
Calcium
Cell Death
Diazepam
Epilepsy
Epilepsy, Temporal Lobe
Fluorescent Antibody Technique
Hippocampus*
Humans
Immunohistochemistry
In Situ Hybridization
Male
Mice*
Microglia
Neurons
Neuropil
Oxidative Stress
Pilocarpine
RNA, Messenger
Seizures
Status Epilepticus*
Up-Regulation
Viola
Calcineurin
Calcium
Diazepam
Pilocarpine
RNA, Messenger
Figure
Reference
-
1. Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J Jr, Forsgren L, French JA, Glynn M, Hesdorffer DC, Lee BI, Mathern GW, Moshé SL, Perucca E, Scheffer IE, Tomson T, Watanabe M, Wiebe S. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014; 55:475–482.
Article2. Blair RD. Temporal lobe epilepsy semiology. Epilepsy Res Treat. 2012; 2012:751510.
Article3. Becker AJ. Review: animal models of acquired epilepsy: insights into mechanisms of human epileptogenesis. Neuropathol Appl Neurobiol. 2018; 44:112–129.
Article4. Hoeffer CA, Dey A, Sachan N, Wong H, Patterson RJ, Shelton JM, Richardson JA, Klann E, Rothermel BA. The Down syndrome critical region protein RCAN1 regulates long-term potentiation and memory via inhibition of phosphatase signaling. J Neurosci. 2007; 27:13161–13172.
Article5. Porta S, Martí E, de la Luna S, Arbonés ML. Differential expression of members of the RCAN family of calcineurin regulators suggests selective functions for these proteins in the brain. Eur J Neurosci. 2007; 26:1213–1226.
Article6. Davies KJ, Ermak G, Rothermel BA, Pritchard M, Heitman J, Ahnn J, Henrique-Silva F, Crawford D, Canaider S, Strippoli P, Carinci P, Min KT, Fox DS, Cunningham KW, Bassel-Duby R, Olson EN, Zhang Z, Williams RS, Gerber HP, Pérez-Riba M, et al. Renaming the DSCR1/Adapt78 gene family as RCAN: regulators of calcineurin. FASEB J. 2007; 21:3023–3028.7. Cano E, Canellada A, Minami T, Iglesias T, Redondo JM. Depolarization of neural cells induces transcription of the Down syndrome critical region 1 isoform 4 via a calcineurin/nuclear factor of activated T cells-dependent pathway. J Biol Chem. 2005; 280:29435–29443.
Article8. Minami T, Horiuchi K, Miura M, Abid MR, Takabe W, Noguchi N, Kohro T, Ge X, Aburatani H, Hamakubo T, Kodama T, Aird WC. Vascular endothelial growth factor- and thrombin-induced termination factor, Down syndrome critical region-1, attenuates endothelial cell proliferation and angiogenesis. J Biol Chem. 2004; 279:50537–50554.
Article9. Yang J, Rothermel B, Vega RB, Frey N, McKinsey TA, Olson EN, Bassel-Duby R, Williams RS. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res. 2000; 87:E61–E68.
Article10. Hesser BA, Liang XH, Camenisch G, Yang S, Lewin DA, Scheller R, Ferrara N, Gerber HP. Down syndrome critical region protein 1 (DSCR1), a novel VEGF target gene that regulates expression of inflammatory markers on activated endothelial cells. Blood. 2004; 104:149–158.
Article11. Wu Y, Song W. Regulation of RCAN1 translation and its role in oxidative stress-induced apoptosis. FASEB J. 2013; 27:208–221.
Article12. Sun X, Wu Y, Herculano B, Song W. RCAN1 overexpression exacerbates calcium overloading-induced neuronal apoptosis. PLoS One. 2014; 9:e95471.
Article13. Fuentes JJ, Genescà L, Kingsbury TJ, Cunningham KW, Pérez-Riba M, Estivill X, de la Luna S. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum Mol Genet. 2000; 9:1681–1690.
Article14. Harris CD, Ermak G, Davies KJ. RCAN1-1L is overexpressed in neurons of Alzheimer's disease patients. FEBS J. 2007; 274:1715–1724.
Article15. Wong H, Levenga J, Cain P, Rothermel B, Klann E, Hoeffer C. RCAN1 overexpression promotes age-dependent mitochondrial dysregulation related to neurodegeneration in Alzheimer's disease. Acta Neuropathol. 2015; 130:829–843.
Article16. Ermak G, Hench KJ, Chang KT, Sachdev S, Davies KJ. Regulator of calcineurin (RCAN1-1L) is deficient in Huntington disease and protective against mutant huntingtin toxicity in vitro. J Biol Chem. 2009; 284:11845–11853.
Article17. Cho KO, Kim YS, Cho YJ, Kim SY. Upregulation of DSCR1 (RCAN1 or Adapt78) in the peri-infarct cortex after experimental stroke. Exp Neurol. 2008; 212:85–92.
Article18. Sobrado M, Ramirez BG, Neria F, Lizasoain I, Arbones ML, Minami T, Redondo JM, Moro MA, Cano E. Regulator of calcineurin 1 (Rcan1) has a protective role in brain ischemia/reperfusion injury. J Neuroinflammation. 2012; 9:48.
Article19. Lin HY, Michtalik HJ, Zhang S, Andersen TT, Van Riper DA, Davies KK, Ermak G, Petti LM, Nachod S, Narayan AV, Bhatt N, Crawford DR. Oxidative and calcium stress regulate DSCR1 (Adapt78/MCIP1) protein. Free Radic Biol Med. 2003; 35:528–539.
Article20. Porta S, Serra SA, Huch M, Valverde MA, Llorens F, Estivill X, Arbonés ML, Martí E. RCAN1 (DSCR1) increases neuronal susceptibility to oxidative stress: a potential pathogenic process in neurodegeneration. Hum Mol Genet. 2007; 16:1039–1050.
Article21. Delorenzo RJ, Sun DA, Deshpande LS. Cellular mechanisms underlying acquired epilepsy: the calcium hypothesis of the induction and maintainance of epilepsy. Pharmacol Ther. 2005; 105:229–266.
Article22. Jeong KH, Lee KE, Kim SY, Cho KO. Upregulation of Krüppel-like factor 6 in the mouse hippocampus after pilocarpine-induced status epilepticus. Neuroscience. 2011; 186:170–178.
Article23. Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972; 32:281–294.
Article24. Jang HJ, Kim JE, Jeong KH, Lim SC, Kim SY, Cho KO. The Neuroprotective effect of Hericium erinaceus extracts in mouse hippocampus after pilocarpine-induced status epilepticus. Int J Mol Sci. 2019; 20:E859.
Article25. Peiris H, Keating DJ. The neuronal and endocrine roles of RCAN1 in health and disease. Clin Exp Pharmacol Physiol. 2018; 45:377–383.
Article26. Wang G, Zhao Y, Liu S, Jia J, Lu T. Critical role of regulator of calcineurin 1 in spinal cord injury. J Physiol Biochem. 2016; 72:605–613.
Article27. Ermak G, Morgan TE, Davies KJ. Chronic overexpression of the calcineurin inhibitory gene DSCR1 (Adapt78) is associated with Alzheimer's disease. J Biol Chem. 2001; 276:38787–38794.
Article28. Canellada A, Ramirez BG, Minami T, Redondo JM, Cano E. Calcium/calcineurin signaling in primary cortical astrocyte cultures: Rcan1-4 and cyclooxygenase-2 as NFAT target genes. Glia. 2008; 56:709–722.
Article29. Hirakawa Y, Nary LJ, Medh RD. Glucocorticoid evoked upregulation of RCAN1-1 in human leukemic CEM cells susceptible to apoptosis. J Mol Signal. 2009; 4:6.
Article30. Peiris H, Dubach D, Jessup CF, Unterweger P, Raghupathi R, Muyderman H, Zanin MP, Mackenzie K, Pritchard MA, Keating DJ. RCAN1 regulates mitochondrial function and increases susceptibility to oxidative stress in mammalian cells. Oxid Med Cell Longev. 2014; 2014:520316.
Article31. Pauletti A, Terrone G, Shekh-Ahmad T, Salamone A, Ravizza T, Rizzi M, Pastore A, Pascente R, Liang LP, Villa BR, Balosso S, Abramov AY, van Vliet EA, Del Giudice E, Aronica E, Patel M, Walker MC, Vezzani A. Targeting oxidative stress improves disease outcomes in a rat model of acquired epilepsy. Brain. 2019; 142:e39.
Article32. Shin EJ, Jeong JH, Chung YH, Kim WK, Ko KH, Bach JH, Hong JS, Yoneda Y, Kim HC. Role of oxidative stress in epileptic seizures. Neurochem Int. 2011; 59:122–137.
Article33. Sun X, Wu Y, Chen B, Zhang Z, Zhou W, Tong Y, Yuan J, Xia K, Gronemeyer H, Flavell RA, Song W. Regulator of calcineurin 1 (RCAN1) facilitates neuronal apoptosis through caspase-3 activation. J Biol Chem. 2011; 286:9049–9062.
Article34. Sun L, Hao Y, An R, Li H, Xi C, Shen G. Overexpression of Rcan1-1L inhibits hypoxia-induced cell apoptosis through induction of mitophagy. Mol Cells. 2014; 37:785–794.
Article35. Kim YS, Cho KO, Lee HJ, Kim SY, Sato Y, Cho YJ. Down syndrome candidate region 1 increases the stability of the IkappaBalpha protein: implications for its anti-inflammatory effects. J Biol Chem. 2006; 281:39051–39061.36. Junkins RD, MacNeil AJ, Wu Z, McCormick C, Lin TJ. Regulator of calcineurin 1 suppresses inflammation during respiratory tract infections. J Immunol. 2013; 190:5178–5186.
Article37. Rana A, Musto AE. The role of inflammation in the development of epilepsy. J Neuroinflammation. 2018; 15:144.
Article38. Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011; 7:31–40.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Time-course changes of hippocalcin expression in the mouse hippocampus following pilocarpine-induced status epilepticus
- Regulator of Calcineurin 1 Isoform 4 (RCAN1.4) Is Overexpressed in the Glomeruli of Diabetic Mice
- Knockdown of RCAN1.4 Increases Susceptibility to FAS-mediated and DNA-damage-induced Apoptosis by Upregulation of p53 Expression
- Neuroprotective effect of lithium after pilocarpine-induced status epilepticus in mice
- Differential Expression of Activating Transcription Factor-2 and c-Jun in the Immature and Adult Rat Hippocampus Following Lithium-Pilocarpine Induced Status Epilepticus