Korean J Physiol Pharmacol.
2017 Jan;21(1):125-131. 10.4196/kjpp.2017.21.1.125.
Neuroprotective effect of lithium after pilocarpine-induced status epilepticus in mice
- Affiliations
-
- 1Department of Physiology, College of Medicine, Dankook University, Cheonan 31116, Korea. heejungkim@dankook.ac.kr
- 2Department of Medical Laser, Graduate School, Dankook University, Cheonan 31116, Korea.
- 3Department of Pharmaceutical Science and Technology, College of Health and Medical Science, Catholic University of Daegu, Gyeongsan 38430, Korea.
- 4Department of Pharmacology, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- KMID: 2371076
- DOI: http://doi.org/10.4196/kjpp.2017.21.1.125
Abstract
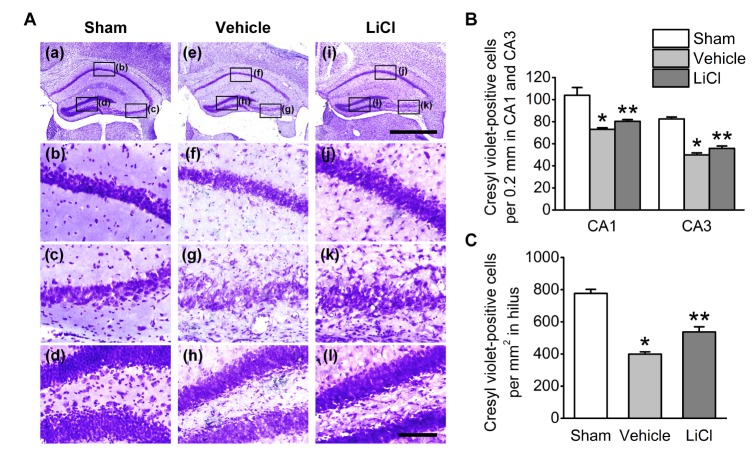
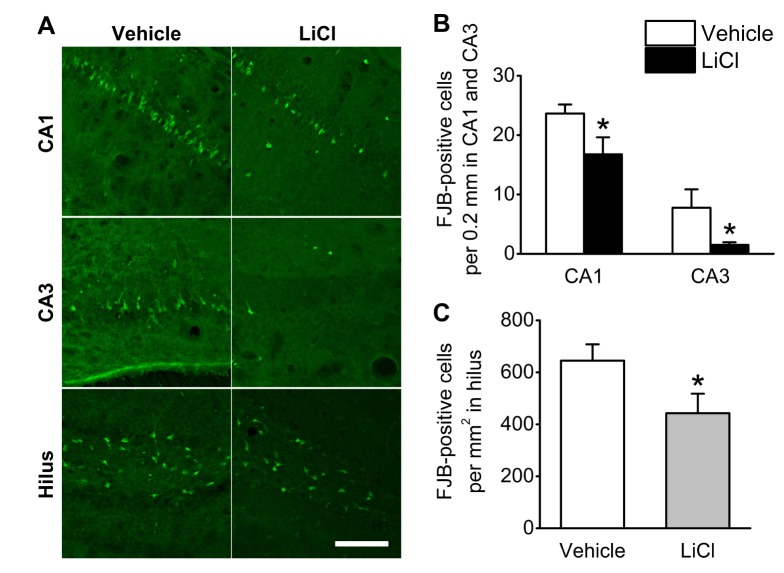
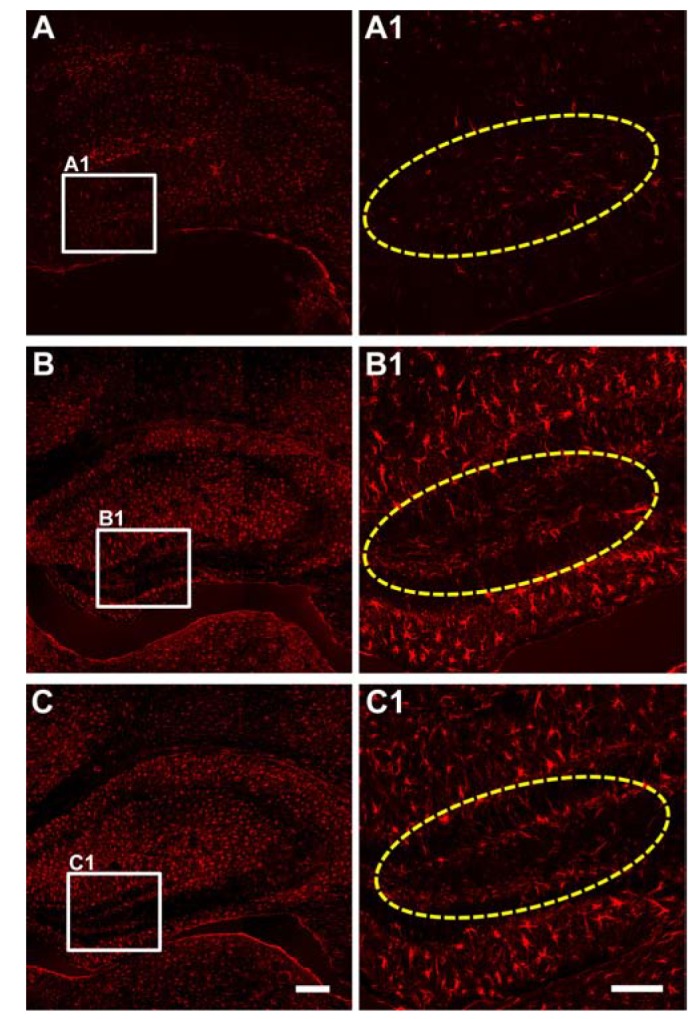
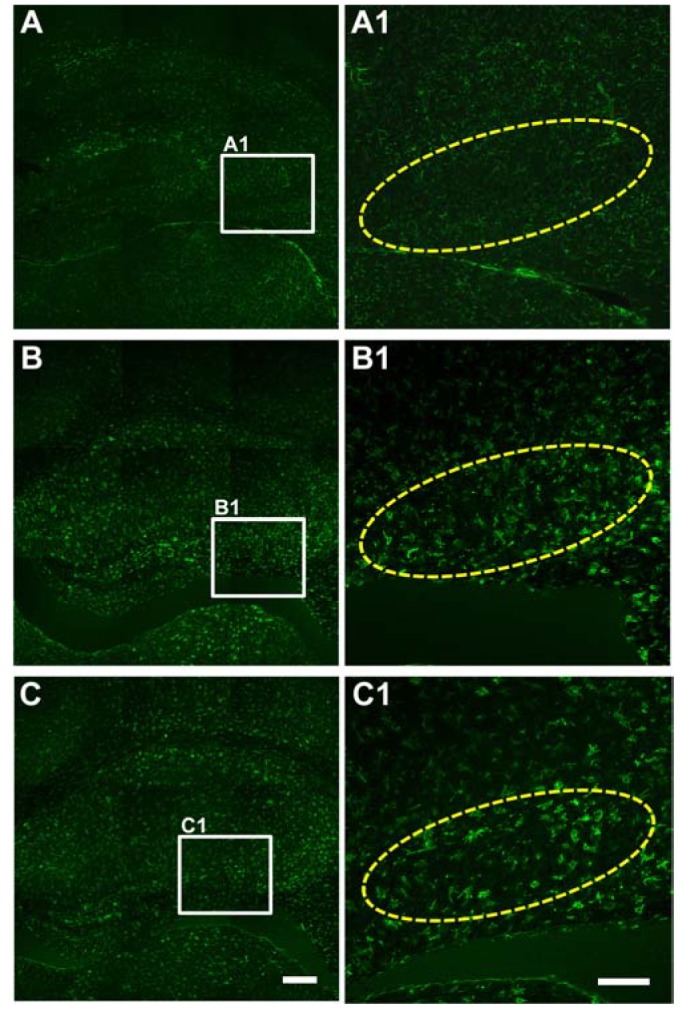
- Status epilepticus is the most common serious neurological condition triggered by abnormal electrical activity, leading to severe and widespread cell loss in the brain. Lithium has been one of the main drugs used for the treatment of bipolar disorder for decades, and its anticonvulsant and neuroprotective properties have been described in several neurological disease models. However, the therapeutic mechanisms underlying lithium's actions remain poorly understood. The muscarinic receptor agonist pilocarpine is used to induce status epilepticus, which is followed by hippocampal damage. The present study was designed to investigate the effects of lithium post-treatment on seizure susceptibility and hippocampal neuropathological changes following pilocarpine-induced status epilepticus. Status epilepticus was induced by administration of pilocarpine hydrochloride (320 mg/kg, i.p.) in C57BL/6 mice at 8 weeks of age. Lithium (80 mg/kg, i.p.) was administered 15 minutes after the pilocarpine injection. After the lithium injection, status epilepticus onset time and mortality were recorded. Lithium significantly delayed the onset time of status epilepticus and reduced mortality compared to the vehicle-treated group. Moreover, lithium effectively blocked pilocarpine-induced neuronal death in the hippocampus as estimated by cresyl violet and Fluoro-Jade B staining. However, lithium did not reduce glial activation following pilocarpine-induced status epilepticus. These results suggest that lithium has a neuroprotective effect and would be useful in the treatment of neurological disorders, in particular status epilepticus.
MeSH Terms
Figure
Reference
-
1. Alldredge BK. Seizure risk associated with psychotropic drugs: clinical and pharmacokinetic considerations. Neurology. 1999; 53(5 Suppl 2):S68–S75. PMID: 10496236.2. Dichter MA. Emerging insights into mechanisms of epilepsy: implications for new antiepileptic drug development. Epilepsia. 1994; 35(Suppl 4):S51–S57.
Article3. Franco V, French JA, Perucca E. Challenges in the clinical development of new antiepileptic drugs. Pharmacol Res. 2016; 103:95–104. PMID: 26611249.
Article4. Licht RW. Lithium: still a major option in the management of bipolar disorder. CNS Neurosci Ther. 2012; 18:219–226. PMID: 22070642.
Article5. Honar H, Riazi K, Homayoun H, Demehri S, Dehghani M, Vafaie K, Ebrahimkhani MR, Rashidi N, Gaskari SA, Dehpour AR. Lithium inhibits the modulatory effects of morphine on susceptibility to pentylenetetrazole-induced clonic seizure in mice: involvement of a nitric oxide pathway. Brain Res. 2004; 1029:48–55. PMID: 15533315.
Article6. Kadlimatti SH, Joseph T. Influence of lithium on the anticonvulsant activity of carbamazepine. Indian J Physiol Pharmacol. 1987; 31:35–41. PMID: 3666873.7. Roy U, Mukherjee BP. Correlation of lithium effect on electroshock-induced seizure in rats with its concentration in brain and plasma. Arch Int Pharmacodyn Ther. 1982; 255:81–88. PMID: 7073401.8. Schmidt J. Lithium suppresses seizure susceptibility in pentylenetetrazol-kindled rats. Biomed Biochim Acta. 1986; 45:1167–1172. PMID: 3028380.9. Nonaka S, Chuang DM. Neuroprotective effects of chronic lithium on focal cerebral ischemia in rats. Neuroreport. 1998; 9:2081–2084. PMID: 9674597.
Article10. Manji HK, Moore GJ, Chen G. Lithium at 50: have the neuroprotective effects of this unique cation been overlooked? Biol Psychiatry. 1999; 46:929–940. PMID: 10509176.
Article11. Müller CJ, Bankstahl M, Gröticke I, Löscher W. Pilocarpine vs. lithium-pilocarpine for induction of status epilepticus in mice: development of spontaneous seizures, behavioral alterations and neuronal damage. Eur J Pharmacol. 2009; 619:15–24. PMID: 19632220.
Article12. Jeong KH, Kim JY, Choi YS, Lee MY, Kim SY. Influence of aspirin on pilocarpine-induced epilepsy in mice. Korean J Physiol Pharmacol. 2013; 17:15–21. PMID: 23439794.
Article13. Choi YS, Lin SL, Lee B, Kurup P, Cho HY, Naegele JR, Lombroso PJ, Obrietan K. Status epilepticus-induced somatostatinergic hilar interneuron degeneration is regulated by striatal enriched protein tyrosine phosphatase. J Neurosci. 2007; 27:2999–3009. PMID: 17360923.
Article14. Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972; 32:281–294. PMID: 4110397.
Article15. Turski L, Ikonomidou C, Turski WA, Bortolotto ZA, Cavalheiro EA. Review: cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy. Synapse. 1989; 3:154–171. PMID: 2648633.
Article16. Dubé C, Boyet S, Marescaux C, Nehlig A. Relationship between neuronal loss and interictal glucose metabolism during the chronic phase of the lithium-pilocarpine model of epilepsy in the immature and adult rat. Exp Neurol. 2001; 167:227–241. PMID: 11161611.
Article17. Lima IV, Campos AC, Bellozi PM, Doria JG, Ribeiro FM, Moraes MF, de Oliveira AC. Postictal alterations induced by intrahippocampal injection of pilocarpine in C57BL/6 mice. Epilepsy Behav. 2016; 64:83–89. PMID: 27736661.
Article18. Drage MG, Holmes GL, Seyfried TN. Hippocampal neurons and glia in epileptic EL mice. J Neurocytol. 2002; 31:681–692. PMID: 14501207.19. Erwin CW, Gerber CJ, Morrison SD, James JF. Lithium carbonate and convulsive disorders. Arch Gen Psychiatry. 1973; 28:646–648. PMID: 4573418.
Article20. Jus A, Villeneuve A, Gautier J, Pires A, Côté JM, Jus K, Villeneuve R, Perron D. Some remarks on the influence of lithium carbonate on patients with temporal epilepsy. Int J Clin Pharmacol. 1973; 7:67–74. PMID: 4699387.21. Jus A, Villeneuve A, Gautier J, Pires A, Côté JM, Jus K, Villeneuve R, Perron D. Influence of lithium carbonate on patients with temporal epilepsy. Can Psychiatr Assoc J. 1973; 18:77–78. PMID: 4571309.
Article22. Tsakiridou E, Bertollini L, de Curtis M, Avanzini G, Pape HC. Selective increase in T-type calcium conductance of reticular thalamic neurons in a rat model of absence epilepsy. J Neurosci. 1995; 15:3110–3117. PMID: 7722649.
Article23. Vreugdenhil M, Wadman WJ. Enhancement of calcium currents in rat hippocampal CA1 neurons induced by kindling epileptogenesis. Neuroscience. 1992; 49:373–381. PMID: 1331856.
Article24. Wasterlain CG, Fujikawa DG, Penix L, Sankar R. Pathophysiological mechanisms of brain damage from status epilepticus. Epilepsia. 1993; 34(Suppl 1):S37–S53. PMID: 8385002.
Article25. Mares P, Mikulecká A. Different effects of two N-methyl-D-aspartate receptor antagonists on seizures, spontaneous behavior, and motor performance in immature rats. Epilepsy Behav. 2009; 14:32–39.26. Gmiro VE, Serdyuk SE. Combined blockade of AMPA and NMDA receptors in the brain of rats prevents pentylenetetrazole-induced clonic and tonic-clonic seizures without ataxia. Bull Exp Biol Med. 2008; 145:728–730. PMID: 19110562.
Article27. Sourial-Bassillious N, Rydelius PA, Aperia A, Aizman O. Glutamate-mediated calcium signaling: a potential target for lithium action. Neuroscience. 2009; 161:1126–1134. PMID: 19362133.
Article28. Payandemehr B, Bahremand A, Ebrahimi A, Nasrabady SE, Rahimian R, Bahremand T, Sharifzadeh M, Dehpour AR. Protective effects of lithium chloride on seizure susceptibility: involvement of α2-adrenoceptor. Pharmacol Biochem Behav. 2015; 133:37–42. PMID: 25824982.
Article29. Ghasemi M, Shafaroodi H, Nazarbeiki S, Meskar H, Heydarpour P, Ghasemi A, Talab SS, Ziai P, Bahremand A, Dehpour AR. Voltage-dependent calcium channel and NMDA receptor antagonists augment anticonvulsant effects of lithium chloride on pentylenetetrazole-induced clonic seizures in mice. Epilepsy Behav. 2010; 18:171–178. PMID: 20605531.
Article30. Bahremand A, Nasrabady SE, Ziai P, Rahimian R, Hedayat T, Payandemehr B, Dehpour AR. Involvement of nitric oxide-cGMP pathway in the anticonvulsant effects of lithium chloride on PTZ-induced seizure in mice. Epilepsy Res. 2010; 89:295–302. PMID: 20304610.
Article31. Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003; 182:21–34. PMID: 12821374.
Article32. Chiu CT, Wang Z, Hunsberger JG, Chuang DM. Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol Rev. 2013; 65:105–142. PMID: 23300133.
Article33. Wada A, Yokoo H, Yanagita T, Kobayashi H. Lithium: potential therapeutics against acute brain injuries and chronic neurodegenerative diseases. J Pharmacol Sci. 2005; 99:307–321. PMID: 16340157.
Article34. Forlenza OV, De-Paula VJ, Diniz BS. Neuroprotective effects of lithium: implications for the treatment of Alzheimer's disease and related neurodegenerative disorders. ACS Chem Neurosci. 2014; 5:443–450. PMID: 24766396.
Article35. Carmichael J, Sugars KL, Bao YP, Rubinsztein DC. Glycogen synthase kinase-3beta inhibitors prevent cellular polyglutamine toxicity caused by the Huntington's disease mutation. J Biol Chem. 2002; 277:33791–33798. PMID: 12097329.36. De Sarno P, Axtell RC, Raman C, Roth KA, Alessi DR, Jope RS. Lithium prevents and ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2008; 181:338–345. PMID: 18566399.
Article37. Watase K, Gatchel JR, Sun Y, Emamian E, Atkinson R, Richman R, Mizusawa H, Orr HT, Shaw C, Zoghbi HY. Lithium therapy improves neurological function and hippocampal dendritic arborization in a spinocerebellar ataxia type 1 mouse model. PLoS Med. 2007; 4:e182. PMID: 17535104.
Article38. Camins A, Verdaguer E, Junyent F, Yeste-Velasco M, Pelegrí C, Vilaplana J, Pallás M. Potential mechanisms involved in the prevention of neurodegenerative diseases by lithium. CNS Neurosci Ther. 2009; 15:333–344. PMID: 19889130.
Article39. Shapiro LA, Wang L, Ribak CE. Rapid astrocyte and microglial activation following pilocarpine-induced seizures in rats. Epilepsia. 2008; 49(Suppl 2):33–41.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antiepileptic and Neuroprotective Effect of Ketamine in Lithium-Pilocarpine Induced Status Epilepticus Rat Model
- The Effect of Corpus Callosotomy in the Lithium-Pilocarpine Induced Status Epileptic Rats
- Differential Expression of Activating Transcription Factor-2 and c-Jun in the Immature and Adult Rat Hippocampus Following Lithium-Pilocarpine Induced Status Epilepticus
- The Effect and the Mechanism of Action of High Frequency Electrical Stimulation of Subthalamic Nucleus on Status Epilepticus Induced by Lithium-Pilocarpine of Rat
- Effect of calcium channel blockers on the hippocampus of Lithium-Pilocarpine induced status epilepticus rat model