Yonsei Med J.
2017 Jan;58(1):27-34. 10.3349/ymj.2017.58.1.27.
Aberrant Hypomethylation of Solute Carrier Family 6 Member 12 Promoter Induces Metastasis of Ovarian Cancer
- Affiliations
-
- 1Department of Biochemistry, School of Medicine, Ewha Womans University, Seoul, Korea. ahnj@ewha.ac.kr
- 2Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, Korea.
- 3College of Pharmacy and Research Institute of Life and Pharmaceutical Sciences, Sunchon National University, Suncheon, Korea.
- 4Department of Obstetrics and Gynecology, School of Medicine, Ewha Womans University, Seoul, Korea. goodmorning@ewha.ac.kr
- KMID: 2374185
- DOI: http://doi.org/10.3349/ymj.2017.58.1.27
Abstract
- PURPOSE
Ovarian cancer (OC) is the most fatal of gynecological malignancies with a high rate of recurrence. We aimed to evaluate the expression of solute carrier family 6, member 12 (SLC6A12) and methylation of its promoter CpG sites in a xenograft mouse model of metastatic OC, and to investigate the regulatory mechanisms that promote aggressive properties during OC progression.
MATERIALS AND METHODS
Expression of SLC6A12 mRNA was determined by reverse-transcription quantitative polymerase chain reaction (RT-qPCR), and DNA methylation status of its promoter CpGs was detected by quantitative methylation-specific PCR. The metastatic potential of SLC6A12 was evaluated by in vitro migration/invasion transwell assays. Gene expression and DNA methylation of SLC6A12 and clinical outcomes were further investigated from publicly available databases from curatedOvarianData and The Cancer Genome Atlas.
RESULTS
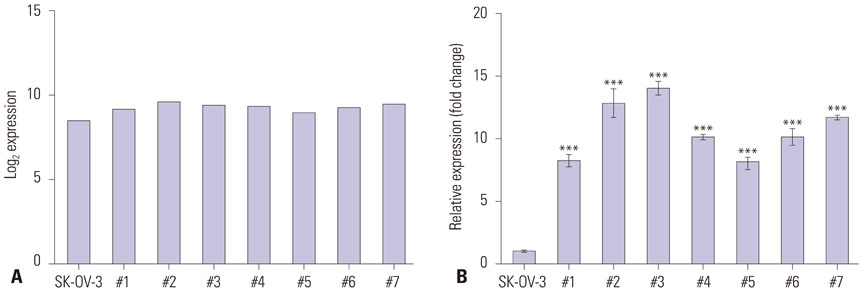
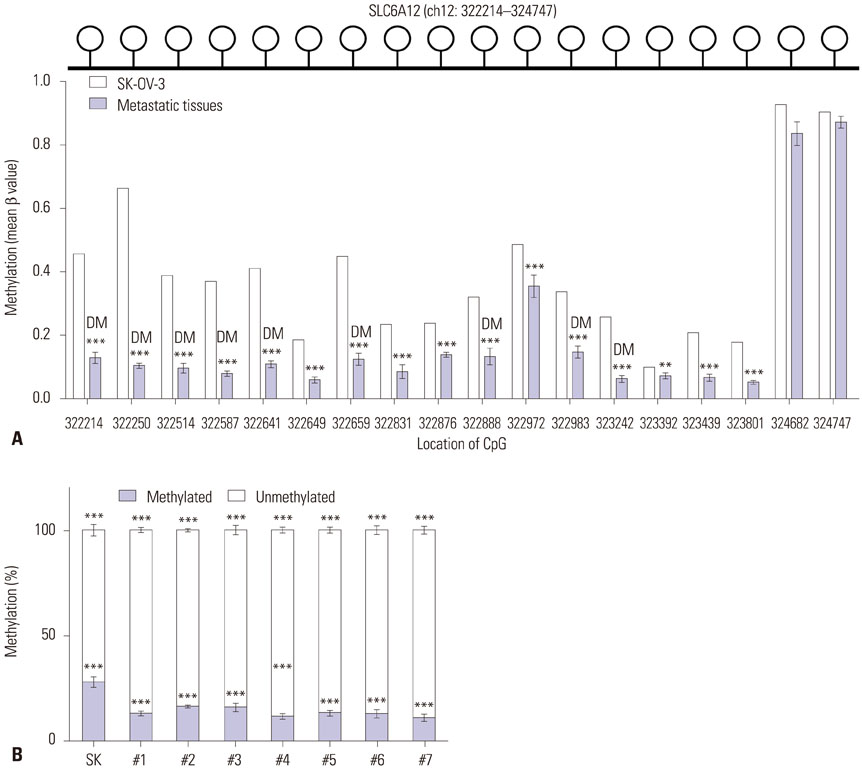
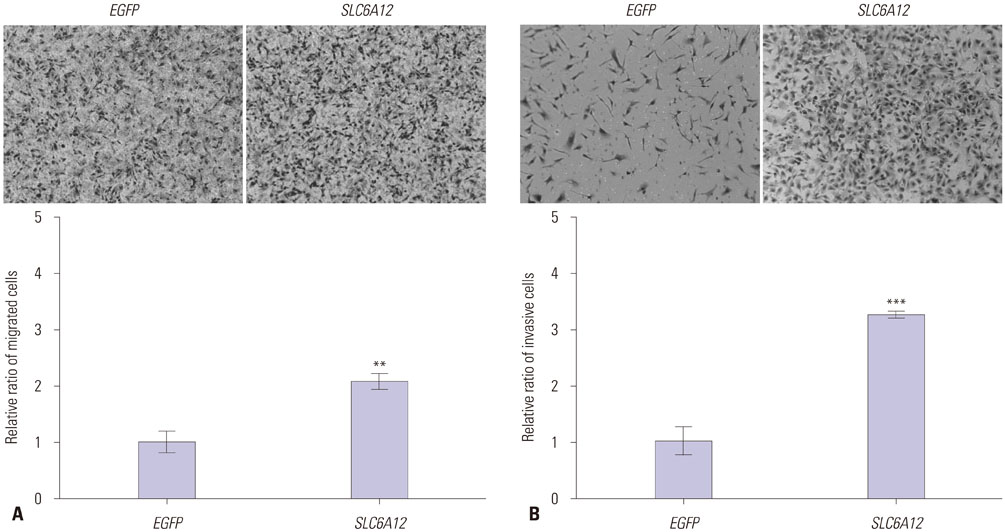
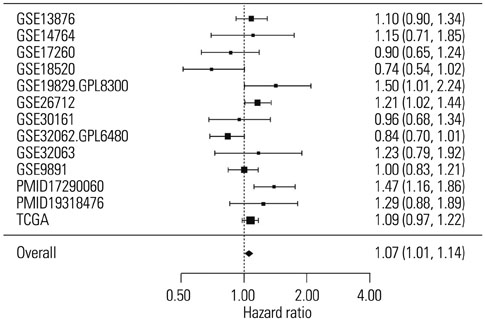
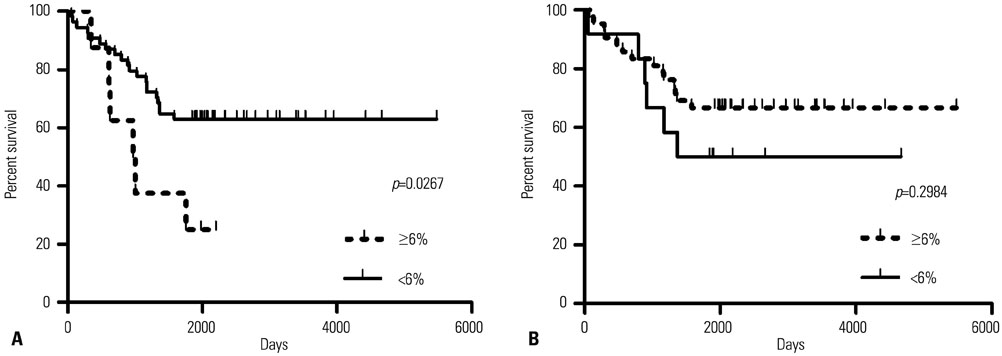
SLC6A12 expression was 8.1-14.0-fold upregulated and its DNA methylation of promoter CpG sites was 41-62% decreased in tumor metastases. After treatment with DNA methyltransferase inhibitor and/or histone deacetylase inhibitor, the expression of SLC6A12 was profoundly enhanced (~8.0-fold), strongly supporting DNA methylation-dependent epigenetic regulation of SLC6A12. Overexpression of SLC6A12 led to increased migration and invasion of ovarian carcinoma cells in vitro, approximately 2.0-fold and 3.3-fold, respectively. The meta-analysis showed that high expression of SLC6A12 was significantly associated with poor overall survival [hazard ratio (HR)=1.07, p value=0.016] and that low DNA methylation levels of SLC6A12 at specific promoter CpG site negatively affected patient survival.
CONCLUSION
Our findings provide novel evidence for the biological and clinical significance of SLC6A12 as a metastasis-promoting gene.
Keyword
MeSH Terms
-
Animals
Carrier Proteins/genetics/*metabolism
Cell Line, Tumor
Cell Migration Assays
*CpG Islands
*DNA Methylation
Disease Progression
Epigenesis, Genetic
Female
Gene Expression Regulation, Neoplastic
Humans
Mice
Neoplasm Invasiveness
Neoplasm Transplantation
Ovarian Neoplasms/genetics/*metabolism/mortality/pathology
Polymerase Chain Reaction
Prognosis
*Promoter Regions, Genetic
RNA, Messenger/*metabolism
Up-Regulation
Carrier Proteins
RNA, Messenger
Figure
Reference
-
1. American Cancer Society. Cancer facts & figures 2015. Atlanta (GA): American Cancer Society;2015.2. Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010; 177:1053–1064.
Article3. Asadollahi R, Hyde CA, Zhong XY. Epigenetics of ovarian cancer: from the lab to the clinic. Gynecol Oncol. 2010; 118:81–87.
Article4. Balch C, Fang F, Matei DE, Huang TH, Nephew KP. Minireview: epigenetic changes in ovarian cancer. Endocrinology. 2009; 150:4003–4011.
Article5. Zhou Y, Holmseth S, Hua R, Lehre AC, Olofsson AM, Poblete-Naredo I, et al. The betaine-GABA transporter (BGT1, SLC6A12) is predominantly expressed in the liver and at lower levels in the kidneys and at the brain surface. Am J Physiol Renal Physiol. 2012; 302:F316–F328.6. Kempson SA, Zhou Y, Danbolt NC. The betaine/GABA transporter and betaine: roles in brain, kidney, and liver. Front Physiol. 2014; 5:159.
Article7. Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004; 3:Article3.
Article8. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995; 289–300.
Article9. Kim NH, Sung HY, Choi EN, Lyu D, Choi HJ, Ju W, et al. Aberrant DNA methylation in the IFITM1 promoter enhances the metastatic phenotype in an intraperitoneal xenograft model of human ovarian cancer. Oncol Rep. 2014; 31:2139–2146.
Article10. Ganzfried BF, Riester M, Haibe-Kains B, Risch T, Tyekucheva S, Jazic I, et al. curatedOvarianData: clinically annotated data for the ovarian cancer transcriptome. Database (Oxford). 2013; 2013:bat013.
Article11. Reimers M, Carey VJ. Bioconductor: an open source framework for bioinformatics and computational biology. Methods Enzymol. 2006; 411:119–134.12. Dugué AE, Pulido M, Chabaud S, Belin L, Gal J. How to deal with interval-censored data practically while assessing the progression-free survival: a step-by-step guide using SAS and R software. Clin Cancer Res. 2016; 09. 12. [Epub]. DOI: 10.1158/1078-0432.CCR-16-1017.
Article13. Zhang D, Li X, Yao Z, Wei C, Ning N, Li J. GABAergic signaling facilitates breast cancer metastasis by promoting ERK1/2-dependent phosphorylation. Cancer Lett. 2014; 348:100–108.
Article14. Wu W, Yang Q, Fung KM, Humphreys MR, Brame LS, Cao A, et al. Linking γ-aminobutyric acid A receptor to epidermal growth factor receptor pathways activation in human prostate cancer. Mol Cell Endocrinol. 2014; 383:69–79.
Article15. Sung HY, Choi EN, Lyu D, Park AK, Ju W, Ahn JH. Aberrant hypomethylation-mediated AGR2 overexpression induces an aggressive phenotype in ovarian cancer cells. Oncol Rep. 2014; 32:815–820.
Article16. Sung HY, Ju W, Ahn JH. DNA hypomethylation-mediated overexpression of carbonic anhydrase 9 induces an aggressive phenotype in ovarian cancer cells. Yonsei Med J. 2014; 55:1656–1663.
Article17. Sung HY, Park AK, Ju W, Ahn JH. Overexpression of mucin 13 due to promoter methylation promotes aggressive behavior in ovarian cancer cells. Yonsei Med J. 2014; 55:1206–1213.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- DNA Hypomethylation-Mediated Overexpression of Carbonic Anhydrase 9 Induces an Aggressive Phenotype in Ovarian Cancer Cells
- The Role of the SLC Transporters Protein in the Neurodegenerative Disorders
- Clinical Study of a Newly Diagnosed Case of Gitelman Syndrome in a Patient Monitored for Liddle Syndrome
- Overexpression of Mucin 13 due to Promoter Methylation Promotes Aggressive Behavior in Ovarian Cancer Cells
- Epigenetic Changes (Aberrant DNA Methylation) in Colorectal Neoplasia