Immune Netw.
2012 Oct;12(5):181-188. 10.4110/in.2012.12.5.181.
Immunomodulatory Effects of Dioscoreae Rhizome Against Inflammation through Suppressed Production of Cytokines Via Inhibition of the NF-kappaB Pathway
- Affiliations
-
- 1College of Pharmacy, Sahmyook University, Seoul 139-742, Korea. kimkj@syu.ac.kr
- 2College of Pharmacy, Chungbuk University, Cheongju 361-763, Korea.
- KMID: 2150747
- DOI: http://doi.org/10.4110/in.2012.12.5.181
Abstract
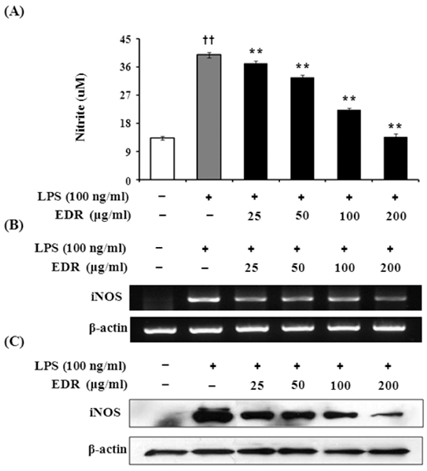
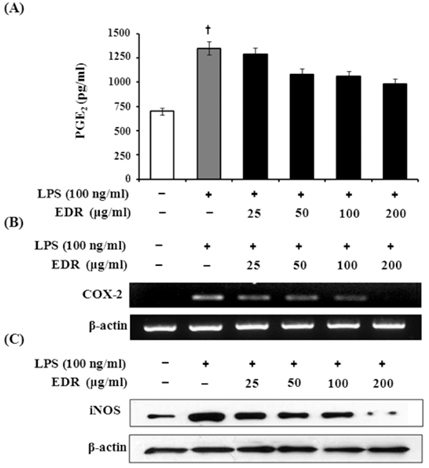
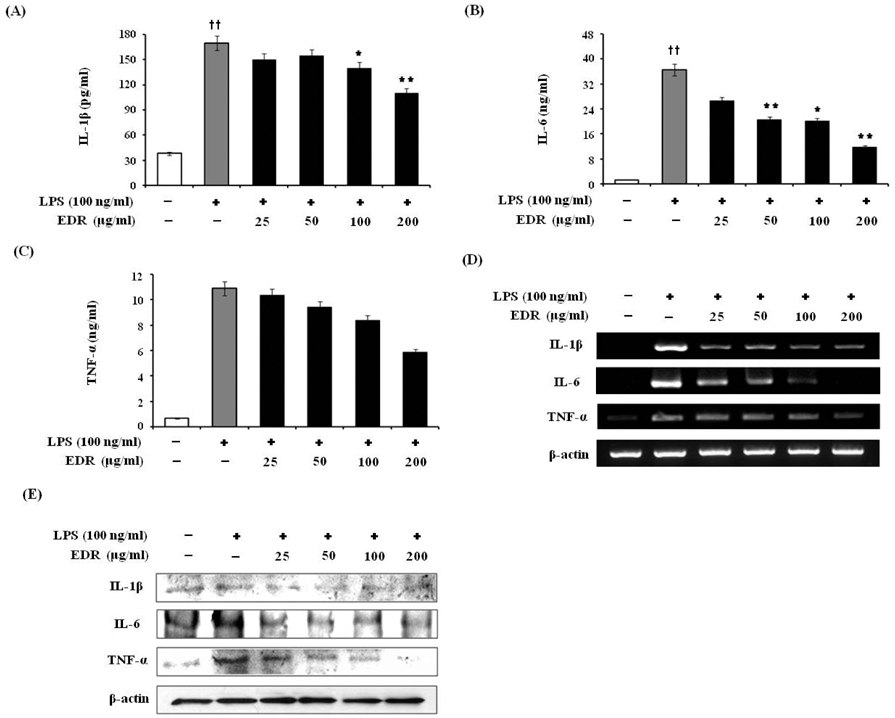
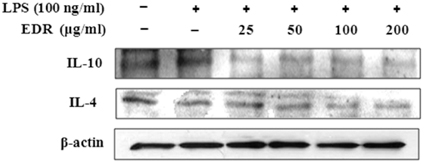
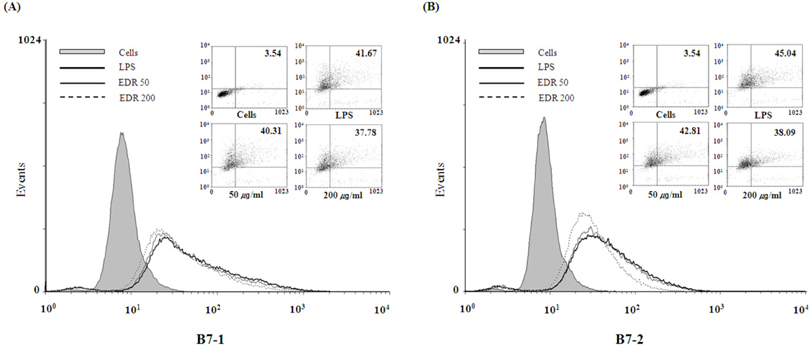
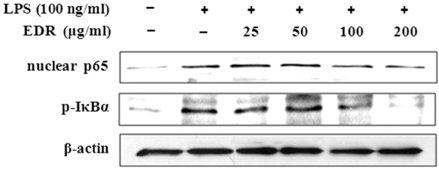
- Dioscoreae Rhizome (DR) has been used in traditional medicine to treat numerous diseases and is reported to have anti-diabetes and anti-tumor activities. To identify a bioactive traditional medicine with anti-inflammatory activity of a water extract of DR (EDR), we determined the mRNA and protein levels of proinflammatory cytokines in macrophages through RT-PCR and western blot analysis and performed a FACS analysis for measuring surface molecules. EDR dose-dependently decreased the production of NO and pro-inflammatory cytokines such as IL-1beta, IL-6, TNF-alpha, and PGE2, as well as mRNA levels of iNOS, COX-2, and pro-inflammatory cytokines, as determined by western blot and RT-PCR analysis, respectively. The expression of co-stimulatory molecules such as B7-1 and B7-2 was also reduced by EDR. Furthermore, activation of the nuclear transcription factor, NF-kappaB, but not that of IL-4 and IL-10, in macrophages was inhibited by EDR. These results show that EDR decreased pro-inflammatory cytokines via inhibition of NF-kappaB-dependent inflammatory protein level, suggesting that EDR could be a useful immunomodulatory agent for treating immunological diseases.
MeSH Terms
-
Blotting, Western
Cytokines
Dinoprostone
Dioscorea
Immune System Diseases
Inflammation
Interleukin-10
Interleukin-4
Interleukin-6
Macrophages
Medicine, Traditional
NF-kappa B
Rhizome
RNA, Messenger
Transcription Factors
Tumor Necrosis Factor-alpha
Water
Cytokines
Dinoprostone
Interleukin-10
Interleukin-4
Interleukin-6
NF-kappa B
RNA, Messenger
Transcription Factors
Tumor Necrosis Factor-alpha
Water
Figure
Reference
-
1. Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G. Atherosclerosis Risk in Communities Study. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003. 52:1799–1805.2. Jacobs M, van Greevenbroek MM, van der Kallen CJ, Ferreira I, Blaak EE, Feskens EJ, Jansen EH, Schalkwijk CG, Stehouwer CD. Low-grade inflammation can partly explain the association between the metabolic syndrome and either coronary artery disease or severity of peripheral arterial disease: the CODAM study. Eur J Clin Invest. 2009. 39:437–444.
Article3. Vane JR, Mitchell JA, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J, Willoughby DA. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci USA. 1994. 91:2046–2050.
Article4. Lee TH, Kwak HB, Kim HH, Lee ZH, Chung DK, Baek NI, Kim J. Methanol extracts of Stewartia koreana inhibit cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) gene expression by blocking NF-kappaB transactivation in LPS-activated RAW 264.7 cells. Mol Cells. 2007. 23:398–404.5. Nathan C. what difference does it make? J Clin Invest. 1997. 100:2417–2423.6. Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001. 2:907–916.
Article7. Vila-del Sol V, Fresno M. Involvement of TNF and NF-kappa B in the transcriptional control of cyclooxygenase-2 expression by IFN-gamma in macrophages. J Immunol. 2005. 174:2825. 2833.
Article8. Lind L. Circulating markers of inflammation and atherosclerosis. Atherosclerosis. 2003. 169:203. 214.
Article9. Tilg H, Wilmer A, Vogel W, Herold M, Nöolchen B, Judmaier G, Huber C. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992. 103:264–274.
Article10. Coker RK, Laurent GJ. Pulmonary fibrosis: cytokines in the balance. Eur Respir J. 1998. 11:1218–1221.
Article11. Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994. 10:405. 455.12. Renard P, Raes M. The proinflammatory transcription factor NFkappaB: a potential target for novel therapeutical strategies. Cell Biol Toxicol. 1999. 15:341–344.13. Tian B, Brasier AR. Identification of a nuclear factor kappa B-dependent gene network. Recent Prog Horm Res. 2003. 58:95–130.
Article14. Zhong X, Nishino E, Okagami N. Temperature dependence of seedling establishment of a perennial, Dioscorea tokoro. J Plant Res. 2002. 115:55–57.
Article15. Lee SC, Tsai CC, Chen JC, Lin CC, Hu ML, Lu S. The evaluation of reno- and hepatoprotective effects of huai-shan-yao (Rhizome Dioscoreae). Am J Chin Med. 2002. 30:609–616.
Article16. Tewtrakul S, Itharat A. Nitric oxide inhibitory substances from the rhizomes of Dioscorea membranacea. J Ethnopharmacol. 2007. 109:412. 416.
Article17. Stuehr DJ, Nathan CF. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989. 169:1543. 1555.
Article18. Lee GI, Ha JY, Min KR, Nakagawa H, Tsurufuji S, Chang IM, Kim Y. Inhibitory effects of oriental herbal medicines on IL-8 induction in lipopolysaccha-rideactivated rat macrophages. Planta Med. 1995. 61:26–30.
Article19. Laskin DL, Pendino KJ. Macrophages and inflammatory mediators in tissue injury. Annu Rev Pharmacol Toxicol. 1995. 35:655–677.
Article20. Furchgott R, Cherry P, Zawadzki J, Jothianandan D. Endothelial cells as mediators of vasodilation of arteries. J Cardiovasc Pharmacol. 1984. 6:S336–S343.
Article21. Wei X, Charles I, Smith A, Ure J, Feng G, Huang H. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995. 375:408–411.
Article22. Ahmad N, Chen LC, Gordon MA, Laskin JD, Laskin DL. Regulation of cyclooxygenase-2 by nitric oxide in activated hepatic macrophages during acute endotoxemia. J Leukoc Biol. 2002. 71:1005–1011.23. Li Q, Verma IM. NFkappa B regulation in the immune system. Nat Rev Immunol. 2002. 2:725–734.24. Richmond A. NF-kappa B, chemokine gene transcription and tumour growth. Nat Rev Immunol. 2002. 2:664. 674.25. Lee S, Shin S, Kim H, Han S, Kim K, Kwon J, Kwak JH, Lee CK, NJ Ha, Yim D, Kim K. Antipin-flammatory function of arctiin by inhibiting COX-2 expression via NF-kappaB pathways. Inflammation. 2011. 8:16.26. Karin M, Ben-Neriah Y. The control of NF-kappa B activity. Annu Rev Immunol. 2000. 18:621–663.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Silymarin Inhibits Morphological Changes in LPS-Stimulated Macrophages by Blocking NF-kappaB Pathway
- Acetylshikonin Inhibits Human Pancreatic PANC-1 Cancer Cell Proliferation by Suppressing the NF-kappaB Activity
- Differential Regulation of NF-kappaB Signaling during Human Cytomegalovirus Infection
- Cordycepin Suppresses Expression of Diabetes Regulating Genes by Inhibition of Lipopolysaccharide-induced Inflammation in Macrophages
- Anticolitic Effect of the Rhizome Mixture of Anemarrhena asphodeloides and Coptidis chinensis (AC-mix) in Mice