Immune Netw.
2009 Jun;9(3):98-105. 10.4110/in.2009.9.3.98.
Cordycepin Suppresses Expression of Diabetes Regulating Genes by Inhibition of Lipopolysaccharide-induced Inflammation in Macrophages
- Affiliations
-
- 1College of Pharmacy, Sahmyook University, Seoul, Korea. kimkj@syu.ac.kr
- 2College of Pharmacy, Chungbuk University, Cheongju, Korea.
- 3Department of Biology,Seoul Women's University, Seoul, Korea.
- KMID: 2150648
- DOI: http://doi.org/10.4110/in.2009.9.3.98
Abstract
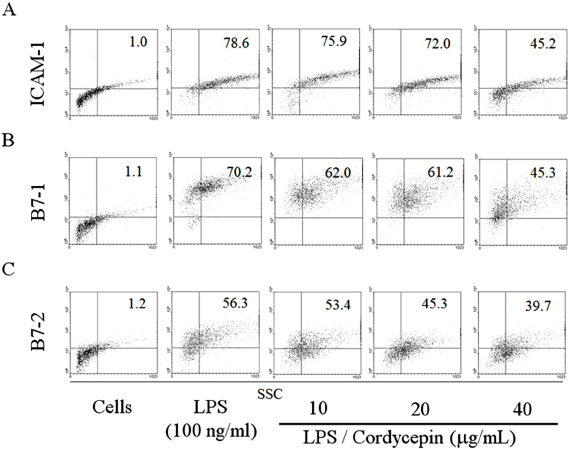
- BACKGROUND: It has been recently noticed that type 2 diabetes (T2D), one of the most common metabolic diseases, causes a chronic low-grade inflammation and activation of the innate immune system that are closely involved in the pathogenesis of T2D. Cordyceps militaris, a traditional medicinal mushroom, produces a component compound, cordycepin (3'-deoxyadenosine). Cordycepin has been known to have many pharmacological activities including immunological stimulating, anti-cancer, and anti-infection activities. The molecular mechanisms of cordycepin in T2D are not clear. In the present study, we tested the role of cordycepin on the anti-diabetic effect and anti-inflammatory cascades in LPS-stimulated RAW 264.7 cells. METHODS: We confirmed the levels of diabetes regulating genes mRNA and protein of cytokines through RT-PCR and western blot analysis and followed by FACS analysis for the surface molecules. RESULTS: Cordycepin inhibited the production of NO and pro-inflammatory cytokines such as IL-1beta, IL-6, and TNF-alpha in LPS-activated macrophages via suppressing protein expression of pro-inflammatory mediators. T2D regulating genes such as 11beta-HSD1 and PPARgamma were decreased as well as expression of co-stimulatory molecules such as ICAM-1 and B7-1/-2 were also decreased with the increment of its concentration. In accordance with suppressed pro-inflammatory cytokine production lead to inhibition of diabetic regulating genes in activated macrophages. Cordycepin suppressed NF-kappaB activation in LPS-activated macrophages. CONCLUSION: Based on these observations, cordycepin suppressed T2D regulating genes through the inactivation of NF-kappaB dependent inflammatory responses and suggesting that cordycepin will provide potential use as an immunomodulatory agent for treating immunological diseases.
MeSH Terms
-
11-beta-Hydroxysteroid Dehydrogenase Type 1
Agaricales
Blotting, Western
Cordyceps
Cytokines
Deoxyadenosines
Immune System
Inflammation
Intercellular Adhesion Molecule-1
Interleukin-6
Macrophages
Metabolic Diseases
NF-kappa B
PPAR gamma
RNA, Messenger
Tumor Necrosis Factor-alpha
11-beta-Hydroxysteroid Dehydrogenase Type 1
Cytokines
Deoxyadenosines
Intercellular Adhesion Molecule-1
Interleukin-6
NF-kappa B
PPAR gamma
RNA, Messenger
Tumor Necrosis Factor-alpha
Figure
Cited by 1 articles
-
Role of Cordycepin and Adenosine on the Phenotypic Switch of Macrophages via Induced Anti-inflammatory Cytokines
Seulmee Shin, Sunhee Moon, Yoonhee Park, Jeonghak Kwon, Seungjeong Lee, Chong-Kil Lee, Kyunghae Cho, Nam-Joo Ha, Kyungjae Kim
Immune Netw. 2009;9(6):255-264. doi: 10.4110/in.2009.9.6.255.
Reference
-
1. Hotamisligil G, Arner P, Caro J, Atkinson R, Spiegelman B. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995. 95:2409–2415.
Article2. Ogden C, Carroll M, Curtin L, McDowell M, Tabak C, Flegal K. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006. 295:1549–1555.
Article3. Li W, Zheng H, Bukuru J, De Kimpe N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol. 2004. 92:1–21.
Article4. DeFronzo R. Pathogenesis of NIDDM. A balanced overview. Diabetes care. 1992. 15:318–368.
Article5. sjöholm A, Nyström T. Endothelial inflammation in insulin resistance. Lancet. 2005. 365:610–612.
Article6. Ross JA, Auger MJ. Burke B, Lewis CE, editors. The biology of the macrophage. The macrophage. 2002. 2nd ed. Oxford: Oxford Medical Publications;1–72.7. Kinne R, Brauer R, Stuhlmuller B, Palombo-Kinne E, Burmester G. Macrophages in rheumatoid arthritis. Arthritis Research. 2000. 2:189–202.8. Gai G, Jin S, Wang B, Li Y, Li C. The efficacy of Cordyceps militaris capsules in treatment of chronic bronchitis in comparison with Jinshuibao capsules. Chin J New Drugs. 2004. 13:169–171.9. Paterson R. Cordyceps: a traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry. 2008. 69:1469–1495.
Article10. Yoo H, Shin J, Cho J, Son C, Lee Y, Park S, Cho C. Effects of Cordyceps militaris extract on angiogenesis and tumor growth. Acta Pharmacologica Sinica. 2004. 25:657–665.11. Kim H, Shrestha B, Lim S, Yoon D, Chang W, Shin D, Han S, Park S, Park J, Park H. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur J Pharmacol. 2006. 545:192–199.
Article12. Yun Y, Han S, Lee S, Ko S, Lee C, Ha N, Kim K. Anti-diabetic effects of CCCA, CMESS, and cordycepin from Cordyceps militaris and the immune responses in streptozotocin-induced diabetic mice. Nat Pro Sci. 2003. 9:291–298.13. Cho M, Lee D, Kim M, Sung J, Ham S. Antimutagenicity and cytotoxicity of cordycepin isolated from Cordyceps militaris. Food Sci Biotechnol. 2003. 12:472–475.14. Majumder N, Dey R, Mathur R, Datta S, Maitra M, Ghosh S, Saha B, Majumdar S. An unusual pro-inflammatory role of interleukin-10 induced by arabinosylated lipoarabinomannan in murine peritoneal macrophages. Glycoconj J. 2006. 23:675–686.
Article15. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004. 25:677–686.
Article16. Furchgott R, Cherry P, Zawadzki J, Jothianandan D. Endothelial cells as mediators of vasodilation of arteries. J Cardiovasc Pharmacol. 1984. 6:S336–S343.
Article17. Rausch-Fan X, Matejka M. From plaque formation to periodontal disease, is there a role for nitric oxide? Eur J Clin Invest. 2008. 31:833–835.
Article18. Wei X, Charles I, Smith A, Ure J, Feng G, Huang F, Xu D, Muller W, Moncada S, Liew F. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995. 375:408–411.
Article19. Haddad JJ, Safieh-Garabedian B, Saadé NE, Kanaan SA, Land SC. Chemioxyexcitation (delta pO2/ROS)-dependent release of IL-1 beta, IL-6 and TNF-alpha: evidence of cytokines as oxygen-sensitive mediators in the alveolar epithelium. Cytokine. 2001. 13:138–147.
Article20. Moestrup S, Moller H. CD163: a regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Annals of Medicine. 2004. 36:347–354.
Article21. Hotamisligil G, Shargill N, Spiegelman B. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993. 259:87–91.
Article22. Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) Induces Insulin Resistance in 3T3-L1 Adipocytes and Is, Like IL-8 and Tumor Necrosis Factor-α, Overexpressed in Human Fat Cells from Insulin-resistant Subjects. J Bio Chem. 2003. 278:45777–45784.
Article23. Uysal KT, Wiesbrock SM, Hotamisligil GS. Functional analysis of tumor necrosis factor (TNF) receptors in TNF-alpha-mediated insulin resistance in genetic obesity. Endocrinology. 1998. 139:4832–4838.
Article24. Xu H, Barnes G, Yang Q, Tan G, Yang D, Chou C, Sole J, Nichols A, Ross J, Tartaglia L. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003. 112:1821–1830.
Article25. Bujalska I, Kumar S, Stewart P. Does central obesity reflect Cushing's disease of the omentum? Lancet. 1997. 349:1210–1213.
Article26. Tomlison J, Stewart P. 11β-Hydroxysteroid dehydrogenase type I as a therapeutic target in the metabolic syndrome. Drug Discov Today. 2005. 2:93–96.27. Wu H, Ghosh S, Perrard X, Feng L, Garcia G, Perrard J, Sweeney J, Peterson L, Chan L, Smith C. T-cell accumulation and regulated on activation, normal T cell expressed secreted upregulation in adipose tissue in obesity. Circulation. 2007. 115:1029.
Article28. Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990. 347:645–650.
Article29. Marx N, Sukhova G, Murphy C, Libby P, Plutzky J. Macrophages in human atheroma contain pparγ differentiation-dependent peroxisomal proliferator-activated receptorγ (PPARγ) expression and reduction of MMP-9 activity through PPARγ activation in mononuclear phagocytes in vitro. ASIP. 1998. 153:17–23.30. Nicklin M, Hughes D, Barton J, Ure J, Duff G. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J Exp Med. 2000. 191:303–312.
Article31. Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, Ikuse T, Asano M, Iwakura Y. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. 2000. 191:313–320.
Article32. Yamshchikov V, Mishina M, Cominelli F. A possible role of IL-1ra 3'-untranslated region in modulation of protein production. Cytokine. 2002. 17:98–107.
Article33. Beach L, Ross J. Cordycepin. An inhibitor of newly synthesized globin messenger RNA. J Bio Chem. 1978. 253:2628–2632.
Article34. Dazzi F, D'andrea E, Biasi G, De Silvestro G, Gaidano G, Schena M, Tison T, Vianello F, Girolami A, Caligaris-Cappio F. Failure of B cells of chronic lymphocytic leukemia in presenting soluble and alloantigens. Clin Immunol Immunopathol. 1995. 75:26–32.
Article35. Deeths MJ, Mescher MF. ICAM-1 and B7-1 provide similar but distinct costimulation for CD8+ T cells, while CD4+ T cells are poorly costimulated by ICAM-1. Eur J Immunol. 1999. 29:45–53.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Cordycepin and Adenosine on the Phenotypic Switch of Macrophages via Induced Anti-inflammatory Cytokines
- Candida spp.- Induced Cytokine Gene Expression on Mouse Peritoneal Macrophages and NIH 3T3 Fibroblasts
- Formosanin C attenuates lipopolysaccharide-induced inflammation through nuclear factor-κB inhibition in macrophages
- Inhibitory Effect of 3-(4-Hydroxyphenyl)-1-(thiophen-2-yl) prop-2-en-1-one, a Chalcone Derivative on MCP-1 Expression in Macrophages via Inhibition of ROS and Akt Signaling
- Pro-Inflammatory Role of S1P₃ in Macrophages