Immune Netw.
2009 Dec;9(6):255-264. 10.4110/in.2009.9.6.255.
Role of Cordycepin and Adenosine on the Phenotypic Switch of Macrophages via Induced Anti-inflammatory Cytokines
- Affiliations
-
- 1College of Pharmacy, Sahmyook University, Seoul 139-742, Korea. kimkj@syu.ac.kr
- 2College of Pharmacy, Chungbuk University, Cheongju 361-763, Korea.
- 3Department of Biology, Seoul Women's University, Seoul 139-774, Korea.
- KMID: 2150654
- DOI: http://doi.org/10.4110/in.2009.9.6.255
Abstract
- BACKGROUND
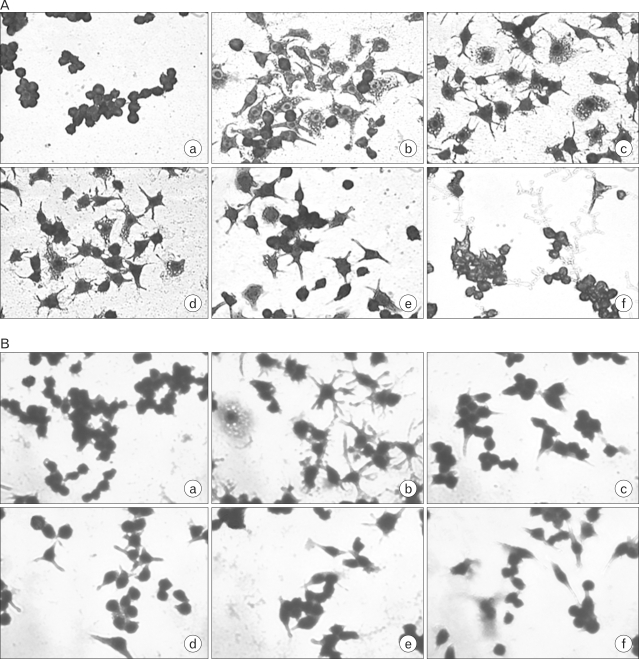
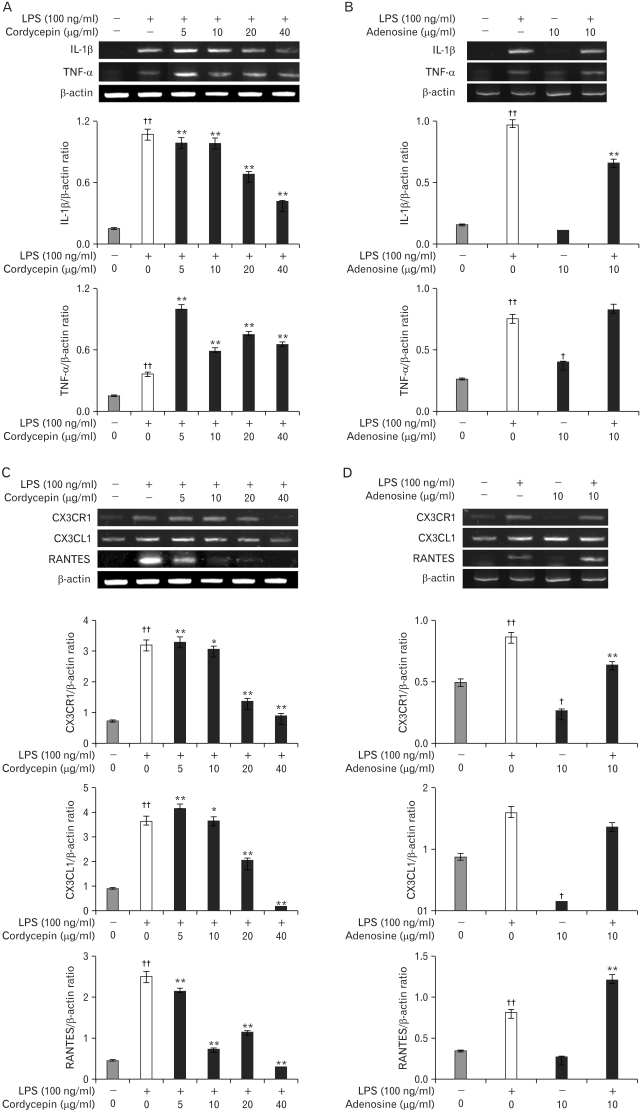
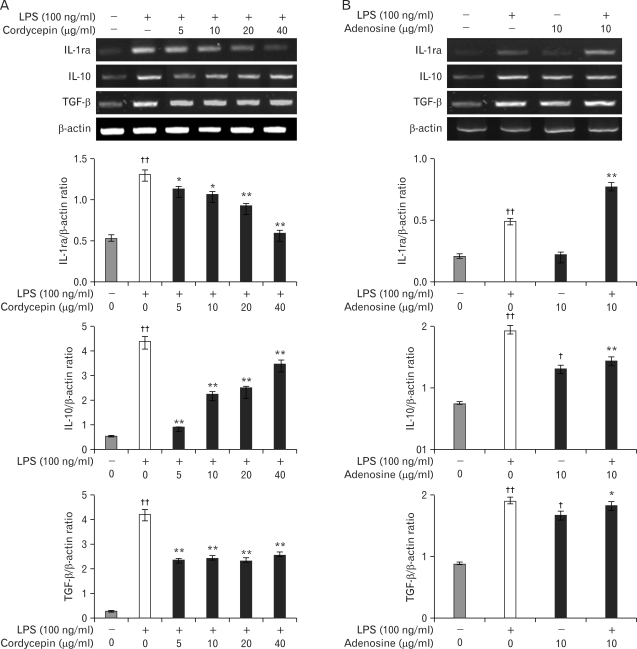
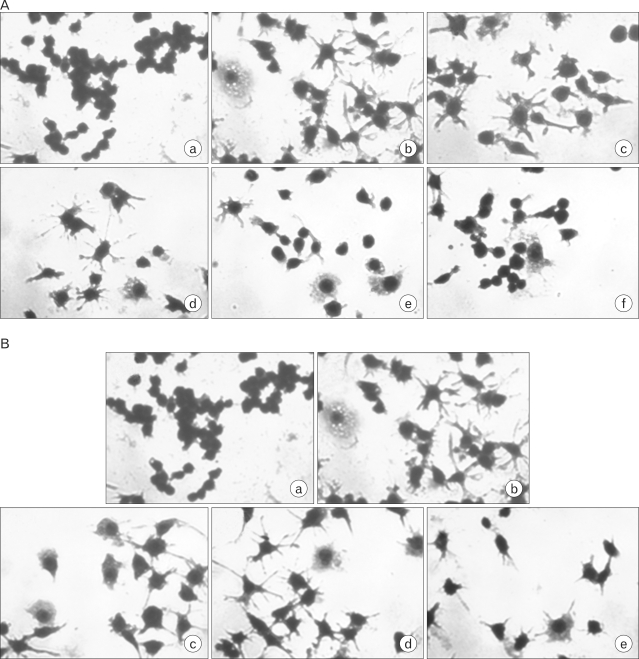
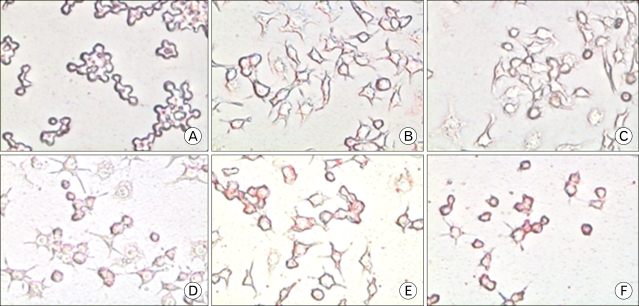
Chronic low grade inflammation is closely linked to type II diabetes, obesity, and atherosclerosis. Macrophages play a key role in the regulation of pro- or anti-inflammatory actions at the lesion sites of disease. Components of cordyceps militaris, cordycepin and adenosine, have been used for the modulation of inflammatory diseases. The effects of cordycepin in the modulation of macrophages have yet to be elucidated. We investigated the effects of cordycepin and adenosine on the morphological changes of macrophages under the inflammatory condition of LPS and an anti-inflammatory condition involving high concentrations of adenosine. METHODS: We confirmed the mRNA levels of the M1/M2 cytokine genes through RT-PCR and morphological change. RESULTS: LPS-activated macrophages returned to their inactivated original shape, i.e., they looked like naive macrophages, through the treatment with high concentrations of cordycepin (40 microgram/ml). LPS and adenosine activated macrophages also returned to their original inactivated shapes after cordycepin treatment; however, at relatively higher levels of cordycepin than adenosine. This change did not occur with relatively low concentrations of cordycepin. Adenosine down-regulated the gene expression of M1 cytokines (IL-1beta, TNF-alpha) and chemokines (CX3CR1, RANTES), as well as cordycepin. Additionally, M2 cytokines (IL-10, IL-1ra, TGF-beta) were up-regulated by both cordycepin and adenosine. CONCLUSION: Based on these observations, both cordycepin and adenosine regulated the phenotypic switch on macrophages and suggested that cordycepin and adenosine may potentially be used as immunomodulatory agents in the treatment of inflammatory disease.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Cordyceps militaris Enhances MHC-restricted Antigen Presentation via the Induced Expression of MHC Molecules and Production of Cytokines
Seulmee Shin, Yoonhee Park, Seulah Kim, Hee-Eun Oh, Young-Wook Ko, Shinha Han, Seungjeong Lee, Chong-Kil Lee, Kyunghae Cho, Kyungjae Kim
Immune Netw. 2010;10(4):135-143. doi: 10.4110/in.2010.10.4.135.Effects of Cordyceps militaris supplementation on the immune response and upper respiratory infection in healthy adults: a randomized, double-blind, placebo-controlled study
Su Jin Jung, Ji Hyun Hwang, Mi Ra Oh, Soo Wan Chae
J Nutr Health. 2019;52(3):258-267. doi: 10.4163/jnh.2019.52.3.258.
Reference
-
1. Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003; 52:1799–1805. PMID: 12829649.2. Jacobs M, van Greevenbroek MM, van der Kallen CJ, Ferreira I, Blaak EE, Feskens EJ, Jansen EH, Schalkwijk CG, Stehouwer CD. Low-grade inflammation can partly explain the association between the metabolic syndrome and either coronary artery disease or severity of peripheral arterial disease: the CODAM study. Eur J Clin Invest. 2009; 39:437–444. PMID: 19397692.
Article3. Gregory JL, Morand EF, McKeown SJ, Ralph JA, Hall P, Yang YH, McColl SR, Hickey MJ. Macrophage migration inhibitory factor induces macrophage recruitment via CC chemokine ligand 2. J Immunol. 2006; 177:8072–8079. PMID: 17114481.
Article4. Schober A, Bernhagen J, Weber C. Chemokine-like functions of MIF in atherosclerosis. J Mol Med. 2008; 86:761–770. PMID: 18385967.
Article5. Mantovani A, Sica A, Locati M. New vistas on macrophage differentiation and activation. Eur J Immunol. 2007; 37:14–16. PMID: 17183610.
Article6. Meghari S, Berruyer C, Lepidi H, Galland F, Naquet P, Mege JL. Vanin-1 controls granuloma formation and macrophage polarization in Coxiella burnetii infection. Eur J Immunol. 2007; 37:24–32. PMID: 17163446.
Article7. Sugar AM, McCaffrey RP. Antifungal activity of 3'-deoxyadenosine (cordycepin). Antimicrob Agents Chemother. 1998; 42:1424–1427. PMID: 9624488.
Article8. Trigg PI, Gutteridge WE, Williamson J. The effects of cordycepin on malaria parasites. Trans R Soc Trop Med Hyg. 1971; 65:514–520. PMID: 4999656.
Article9. de Julián-Ortiz JV, Gálvez J, Muñoz-Collado C, García-Domenech R, Gimeno-Cardona C. Virtual combinatorial syntheses and computational screening of new potential anti-herpes compounds. J Med Chem. 1999; 42:3308–3314. PMID: 10464017.
Article10. Deitch AD, Sawicki SG. Effects of cordycepin on microtubules of cultured mammalian cells. Exp Cell Res. 1979; 118:1–13. PMID: 310391.
Article11. Yun Y, Han S, Lee S, Ko SK, Lee C, Ha NJ, Kim K. Anti-diabetic effects of CCCA, CMESS, and cordycepin from Cordyceps militaris and the immune responses in streptozotocin-induced diabetic mice. Nat Prod Sci. 2003; 9:291–298.12. Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998; 50:413–492. PMID: 9755289.13. Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International union of pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001; 53:527–552. PMID: 11734617.14. Drury AN, Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929; 68:213–237. PMID: 16994064.15. Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003; 73:209–212. PMID: 12554797.
Article16. Haddad JJ, Safieh-Garabedian B, Saadé NE, Kanaan SA, Land SC. Chemioxyexcitation (delta pO2/ROS)-dependent release of IL-1 beta, IL-6 and TNF-alpha: evidence of cytokines as oxygen-sensitive mediators in the alveolar epithelium. Cytokine. 2001; 13:138–147. PMID: 11161456.17. Shin S, Lee S, Kwon J, Moon S, Lee S, Lee CK, Cho K, Ha NJ, Kim K. Cordycepin suppresses expression of diabetes regulating genes by inhibition of lipopolysaccharide-induced inflammation in macrophages. Immune Netw. 2009; 9:98–105. PMID: 20107539.
Article18. Kim HG, Shrestha B, Lim SY, Yoon DH, Chang WC, Shin DJ, Han SK, Park SM, Park JH, Park HI, Sung JM, Jang Y, Chung N, Hwang KC, Kim TW. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-κB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur J Pharmacol. 2006; 545:192–199. PMID: 16899239.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cordycepin Suppresses Expression of Diabetes Regulating Genes by Inhibition of Lipopolysaccharide-induced Inflammation in Macrophages
- Production of IFN-gamma by TNF-alpha in Macrophages from Tumor Micro Environment; Significance in Angiogenic Switch Control
- Differential Activation of Macrophages Based on Their Environment in Advanced Age
- Macrophages and Inflammation
- Effect of Cordycepin-Enriched WIB801C from Cordyceps militaris Suppressing Fibrinogen Binding to Glycoprotein IIb/IIIa