Chonnam Med J.
2020 Jan;56(1):12-19. 10.4068/cmj.2020.56.1.12.
Differential Activation of Macrophages Based on Their Environment in Advanced Age
- Affiliations
-
- 1Department of Biochemistry, Chonnam National University Medical School, Hwasun, Korea. dr.jslim7542@gmail.com, kacho@jnu.ac.kr
- 2Center for Creative Biomedical Scientists, Chonnam National University Medical School, Hwasun, Korea.
- 3Combinatorial Tumor Immunotherapy Medical Research Center, Chonnam National University Medical School, Hwasun, Korea.
- KMID: 2468141
- DOI: http://doi.org/10.4068/cmj.2020.56.1.12
Abstract
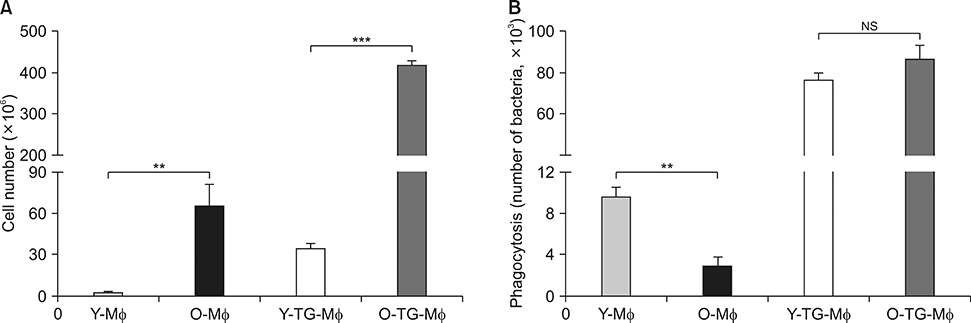
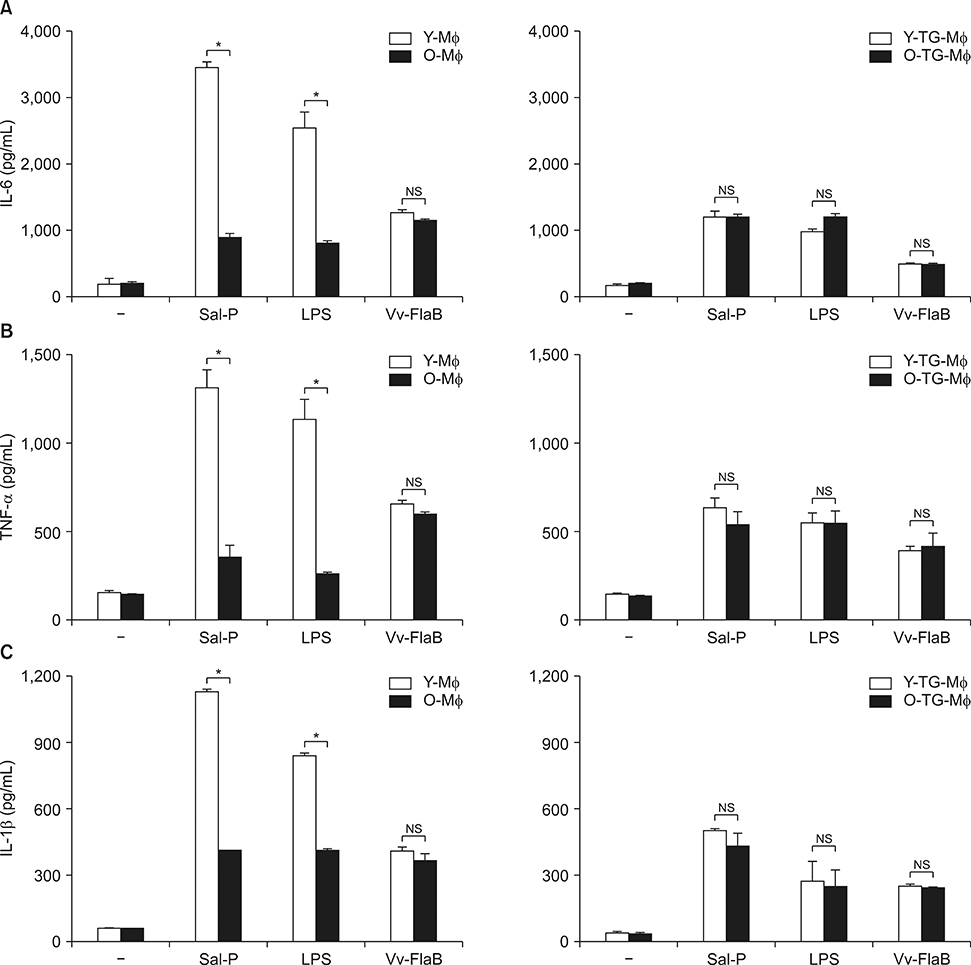
- The macrophage displays functional and phenotypic diversity, which appears, in no small part, to stem from the ability of macrophages to adapt functionally to changes in their tissue microenvironment. Here, we describe the differential activity of peritoneal macrophages with or without the presence of thioglycollate (TG), an inflammatory drug that encouraged the recruitment of macrophages, during aging. The peritoneal-resident macrophages dramatically reduced in phagocytosis and pro-inflammatory cytokines secretion with aging, whereas the functions of macrophages recruited by TG were not significantly changed with aging. These results suggest that macrophages may be changed by their environment in advanced age, and could provide possible explanations for the controversial results regarding differential changes in macrophages in other papers.
Keyword
Figure
Reference
-
1. Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol. 2018; 9:586.
Article2. Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013; 14:877–882.
Article3. Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016; 529:307–315.
Article4. Oishi Y, Manabe I. Macrophages in age-related chronic inflammatory diseases. NPJ Aging Mech Dis. 2016; 2:16018.
Article5. Licastro F, Candore G, Lio D, Porcellini E, Colonna-Romano G, Franceschi C, Caruso C. Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun Ageing. 2005; 2:8.
Article6. O'Neill L. Toll-like receptors and the danger hypothesis. Trends Immunol. 2001; 22:421.7. Austyn JM. Death, destruction, danger and dendritic cells. Nat Med. 1999; 5:1232–1233.
Article8. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010; 327:656–661.
Article9. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011; 11:723–737.
Article10. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014; 5:514.
Article11. Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011; 11:738–749.
Article12. Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009; 27:451–483.
Article13. Stout RD, Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol Rev. 2005; 205:60–71.
Article14. Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000; 908:244–254.
Article15. Saurwein-Teissl M, Blasko I, Zisterer K, Neuman B, Lang B, Grubeck-Loebenstein B. An imbalance between pro- and anti-inflammatory cytokines, a characteristic feature of old age. Cytokine. 2000; 12:1160–1161.
Article16. Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002; 169:4697–4701.
Article17. Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004; 75:342–349.
Article18. Lim JS, Nguyen KC, Nguyen CT, Jang IS, Han JM, Fabian C, et al. Flagellin-dependent TLR5/caveolin-1 as a promising immune activator in immunosenescence. Aging Cell. 2015; 14:907–915.
Article19. Jeong JH, Kim K, Lim D, Kim KH, Kim HS, Lee S, et al. Microvasculature remodeling in the mouse lower gut during inflammaging. Sci Rep. 2017; 7:39848.
Article20. Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010; 10:36–46.
Article21. Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006; 236:13–23.
Article22. Jiménez-Dalmaroni MJ, Gerswhin ME, Adamopoulos IE. The critical role of toll-like receptors--from microbial recognition to autoimmunity: a comprehensive review. Autoimmun Rev. 2016; 15:1–8.
Article23. Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014; 5:614.
Article24. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008; 8:958–969.
Article25. Gordon S, Plüddemann A, Martinez Estrada F. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev. 2014; 262:36–55.
Article26. Fietta A, Merlini C, De Bernardi PM, Gandola L, Piccioni PD, Grassi C. Non specific immunity in aged healthy subjects and in patients with chronic bronchitis. Aging (Milano). 1993; 5:357–361.
Article27. Hilmer SN, Cogger VC, Le Couteur DG. Basal activity of Kupffer cells increases with old age. J Gerontol A Biol Sci Med Sci. 2007; 62:973–978.
Article28. Miller AP, Xing D, Feng W, Fintel M, Chen YF, Oparil S. Aged rats lose vasoprotective and anti-inflammatory actions of estrogen in injured arteries. Menopause. 2007; 14:251–260.
Article29. Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004; 3:161–167.
Article30. Chelvarajan RL, Liu Y, Popa D, Getchell ML, Getchell TV, Stromberg AJ, et al. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J Leukoc Biol. 2006; 79:1314–1327.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of Macrophage Activation and Differentiation in Atherosclerosis
- The Clinical Impact of the Dendritic Cell-based Cancer Vaccine: the Role in the Inflammatory Tumor Micro-environment
- Comparison of Macrophage Activation and Tumor - cytotoxicity in Mouse and hamster Peritoneal Macrophages by Cold Stress
- Non-specific activation of mouse peritoneal macrophages by a freshwater ciliate, Tetrahymena pyriformis
- Effects of cytokines in the activation of peritoneal macrophages from mice infected with Toxoplasma gondii