Korean J Physiol Pharmacol.
2015 May;19(3):211-218. 10.4196/kjpp.2015.19.3.211.
Silymarin Inhibits Morphological Changes in LPS-Stimulated Macrophages by Blocking NF-kappaB Pathway
- Affiliations
-
- 1Department of Pharmacology, School of Medicine, Chosun University, Gwangju 501-759, Korea. yjjeon@chosun.ac.kr
- KMID: 1791421
- DOI: http://doi.org/10.4196/kjpp.2015.19.3.211
Abstract
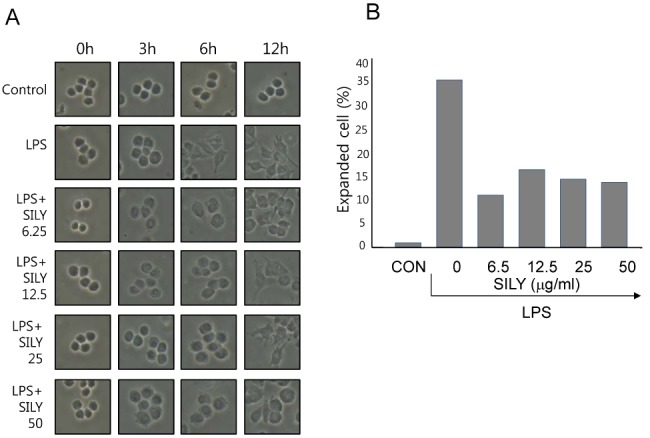
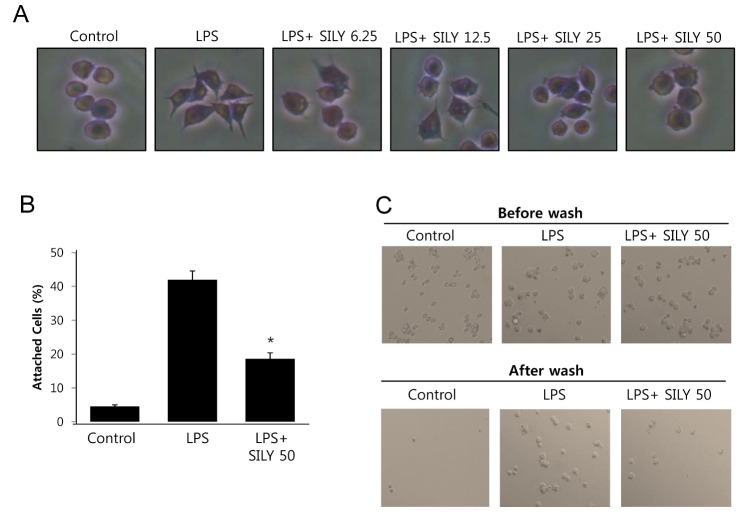
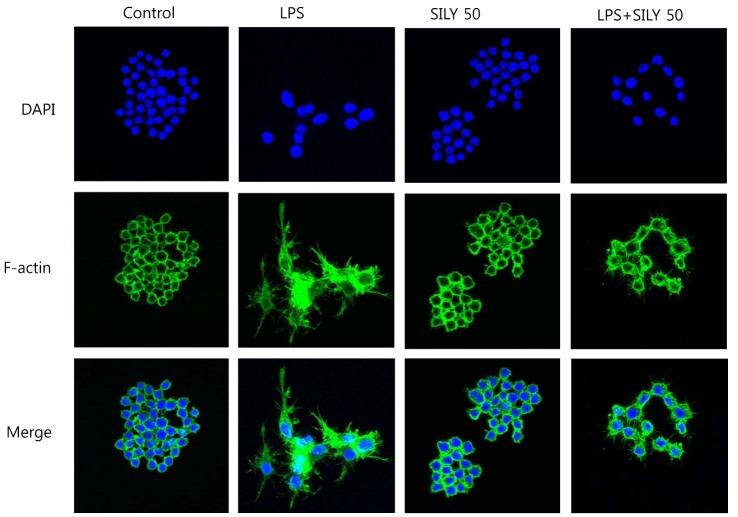
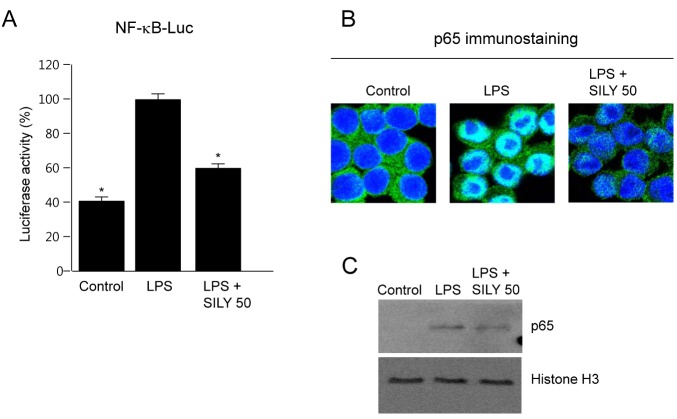
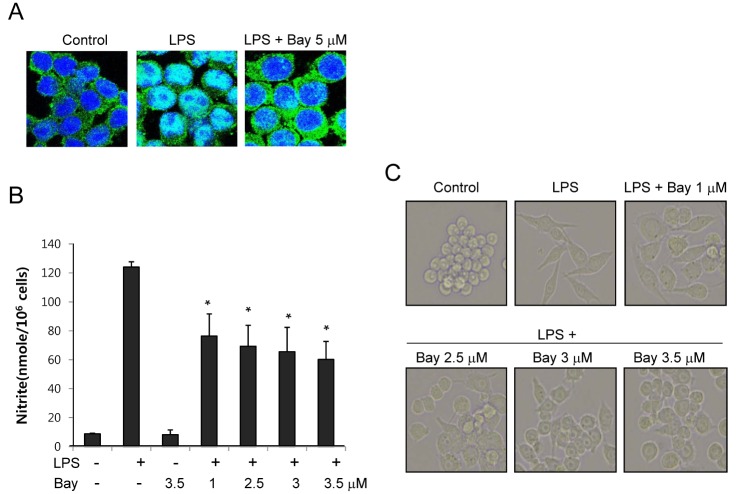
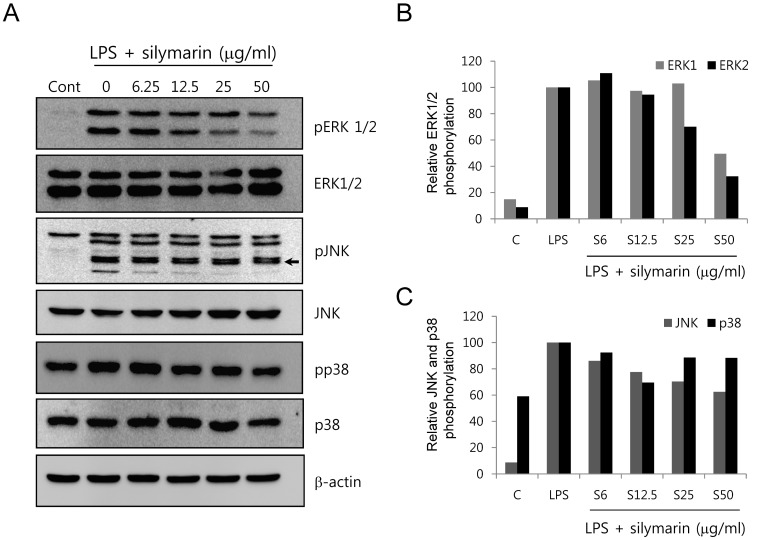
- The present study showed that silymarin, a polyphenolic flavonoid isolated from milk thistle (Silybum marianum), inhibited lipopolysaccharide (LPS)-induced morphological changes in the mouse RAW264.7 macrophage cell line. We also showed that silymarin inhibited the nuclear translocation and transactivation activities of nuclear factor-kappa B (NF-kappaB), which is important for macrophage activation-associated changes in cell morphology and gene expression of inflammatory cytokines. BAY-11-7085, an NF-kappaB inhibitor, abrogated LPS-induced morphological changes and NO production, similar to silymarin. Treatment of RAW264.7 cells with silymarin also inhibited LPS-stimulated activation of mitogen-activated protein kinases (MAPKs). Collectively, these experiments demonstrated that silymarin inhibited LPS-induced morphological changes in the RAW264.7 mouse macrophage cell line. Our findings indicated that the most likely mechanism underlying this biological effect involved inhibition of the MAPK pathway and NF-kappaB activity. Inhibition of these activities by silymarin is a potentially useful strategy for the treatment of inflammation because of the critical roles played by MAPK and NF-kappaB in mediating inflammatory responses in macrophages.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Carnosic acid protects against acetaminophen-induced hepatotoxicity by potentiating Nrf2-mediated antioxidant capacity in mice
Qi Guo, Zhiyang Shen, Hongxia Yu, Gaofeng Lu, Yong Yu, Xia Liu, Pengyuan Zheng
Korean J Physiol Pharmacol. 2016;20(1):15-23. doi: 10.4196/kjpp.2016.20.1.15.
Reference
-
1. Radjadian T, Rezazadeh SH, Huseini HF. Analysis of silymarin components in the seed extracts of some milk thistle ecotypes from Iran by Hplc. Iranian Journal of Science and Technology Transaction a-Science. 2008; 32:141–146.2. Valenzuela A, Garrido A. Biochemical bases of the pharmacological action of the flavonoid silymarin and of its structural isomer silibinin. Biol Res. 1994; 27:105–112. PMID: 8640239.3. Zhang W, Hong R, Tian T. Silymarin's Protective effects and possible mechanisms on alcoholic fatty liver for rats. Biomol Ther (Seoul). 2013; 21:264–269. PMID: 24244810.
Article4. Bektur NE, Sahin E, Baycu C, Unver G. Protective effects of silymarin against acetaminophen-induced hepatotoxicity and nephrotoxicity in mice. Toxicol Ind Health. 2013; [Epub ahead of print].
Article5. Mereish KA, Bunner DL, Ragland DR, Creasia DA. Protection against microcystin-LR-induced hepatotoxicity by Silymarin: biochemistry, histopathology, and lethality. Pharm Res. 1991; 8:273–277. PMID: 1902564.6. Kvasnicka F, Bíba B, Sevcík R, Voldrich M, Krátká J. Analysis of the active components of silymarin. J Chromatogr A. 2003; 990:239–245. PMID: 12685603.7. Cristofalo R, Bannwart-Castro CF, Magalhães CG, Borges VT, Peraçoli JC, Witkin SS, Peraçoli MT. Silibinin attenuates oxidative metabolism and cytokine production by monocytes from preeclamptic women. Free Radic Res. 2013; 47:268–275. PMID: 23316804.
Article8. Kang JS, Jeon YJ, Kim HM, Han SH, Yang KH. Inhibition of inducible nitric-oxide synthase expression by silymarin in lipopolysaccharide-stimulated macrophages. J Pharmacol Exp Ther. 2002; 302:138–144. PMID: 12065710.
Article9. Mokhtari MJ, Motamed N, Shokrgozar MA. Evaluation of silibinin on the viability, migration and adhesion of the human prostate adenocarcinoma (PC-3) cell line. Cell Biol Int. 2008; 32:888–892. PMID: 18538589.
Article10. Hogan FS, Krishnegowda NK, Mikhailova M, Kahlenberg MS. Flavonoid, silibinin, inhibits proliferation and promotes cellcycle arrest of human colon cancer. J Surg Res. 2007; 143:58–65. PMID: 17950073.
Article11. Ahmed-Belkacem A, Ahnou N, Barbotte L, Wychowski C, Pallier C, Brillet R, Pohl RT, Pawlotsky JM. Silibinin and related compounds are direct inhibitors of hepatitis C virus RNAdependent RNA polymerase. Gastroenterology. 2010; 138:1112–1122. PMID: 19962982.
Article12. Pferschy-Wenzig EM, Atanasov AG, Malainer C, Noha SM, Kunert O, Schuster D, Heiss EH, Oberlies NH, Wagner H, Bauer R, Dirsch VM. Identification of isosilybin a from milk thistle seeds as an agonist of peroxisome proliferator-activated receptor gamma. J Nat Prod. 2014; 77:842–847. PMID: 24597776.
Article13. Lettéron P, Labbe G, Degott C, Berson A, Fromenty B, Delaforge M, Larrey D, Pessayre D. Mechanism for the protective effects of silymarin against carbon tetrachloride-induced lipid peroxidation and hepatotoxicity in mice. Evidence that silymarin acts both as an inhibitor of metabolic activation and as a chain-breaking antioxidant. Biochem Pharmacol. 1990; 39:2027–2034. PMID: 2353942.14. Zhao J, Sharma Y, Agarwal R. Significant inhibition by the flavonoid antioxidant silymarin against 12-O-tetradecanoylphorbol 13-acetate-caused modulation of antioxidant and inflammatory enzymes, and cyclooxygenase 2 and interleukin-1alpha expression in SENCAR mouse epidermis: implications in the prevention of stage I tumor promotion. Mol Carcinog. 1999; 26:321–333. PMID: 10569809.15. Singh RP, Tyagi AK, Zhao J, Agarwal R. Silymarin inhibits growth and causes regression of established skin tumors in SENCAR mice via modulation of mitogen-activated protein kinases and induction of apoptosis. Carcinogenesis. 2002; 23:499–510. PMID: 11895866.
Article16. Kim S, Choi MG, Lee HS, Lee SK, Kim SH, Kim WW, Hur SM, Kim JH, Choe JH, Nam SJ, Yang JH, Kim S, Lee JE, Kim JS. Silibinin suppresses TNF-alpha-induced MMP-9 expression in gastric cancer cells through inhibition of the MAPK pathway. Molecules. 2009; 14:4300–4311. PMID: 19924065.17. Kim JH, Kim K, Jin HM, Song I, Youn BU, Lee J, Kim N. Silibinin inhibits osteoclast differentiation mediated by TNF family members. Mol Cells. 2009; 28:201–207. PMID: 19756392.
Article18. Pi J, Li T, Liu J, Su X, Wang R, Yang F, Bai H, Jin H, Cai J. Detection of lipopolysaccharide induced inflammatory responses in RAW264.7 macrophages using atomic force microscope. Micron. 2014; 65:1–9. PMID: 25041825.
Article19. Machesky LM. Lamellipodia and filopodia in metastasis and invasion. FEBS Lett. 2008; 582:2102–2111. PMID: 18396168.
Article20. Bhavsar PJ, Vigorito E, Turner M, Ridley AJ. Vav GEFs regulate macrophage morphology and adhesion-induced Rac and Rho activation. Exp Cell Res. 2009; 315:3345–3358. PMID: 19715691.
Article21. Guo AK, Hou YY, Hirata H, Yamauchi S, Yip AK, Chiam KH, Tanaka N, Sawada Y, Kawauchi K. Loss of p53 enhances NF-κ B-dependent lamellipodia formation. J Cell Physiol. 2014; 229:696–704. PMID: 24647813.22. Kucik DF, Wu C. Cell-adhesion assays. Methods Mol Biol. 2005; 294:43–54. PMID: 15576904.
Article23. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982; 126:131–138. PMID: 7181105.
Article24. Huong PT, Lee MY, Lee KY, Chang IY, Lee SK, Yoon SP, Lee DC, Jeon YJ. Synergistic Induction of iNOS by IFN-γ and Glycoprotein Isolated from Dioscorea batatas. Korean J Physiol Pharmacol. 2012; 16:431–436. PMID: 23269906.25. Kim EJ, Kim J, Lee MY, Sudhanva MS, Devakumar S, Jeon YJ. Silymarin inhibits cytokine-stimulated pancreatic beta cells by blocking the ERK1/2 pathway. Biomol Ther (Seoul). 2014; 22:282–287. PMID: 25143805.
Article26. Hibbs JB Jr, Taintor RR, Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987; 235:473–476. PMID: 2432665.
Article27. Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988; 333:664–666. PMID: 3131684.
Article28. Matsuda T, Ferreri K, Todorov I, Kuroda Y, Smith CV, Kandeel F, Mullen Y. Silymarin protects pancreatic beta-cells against cytokine-mediated toxicity: implication of c-Jun NH2-terminal kinase and janus kinase/signal transducer and activator of transcription pathways. Endocrinology. 2005; 146:175–185. PMID: 15459112.29. Cetkovic-Cvrlje M, Eizirik DL. TNF-alpha and IFN-gamma potentiate the deleterious effects of IL-1 beta on mouse pancreatic islets mainly via generation of nitric oxide. Cytokine. 1994; 6:399–406. PMID: 7948748.30. Darville MI, Eizirik DL. Regulation by cytokines of the inducible nitric oxide synthase promoter in insulin-producing cells. Diabetologia. 1998; 41:1101–1108. PMID: 9754830.
Article31. Soto C, Raya L, Juárez J, Pérez J, González I. Effect of Silymarin in Pdx-1 expression and the proliferation of pancreatic β-cells in a pancreatectomy model. Phytomedicine. 2014; 21:233–239. PMID: 24176839.
Article32. Agarwal R, Agarwal C, Ichikawa H, Singh RP, Aggarwal BB. Anticancer potential of silymarin: from bench to bed side. Anticancer Res. 2006; 26:4457–4498. PMID: 17201169.33. Iademarco MF, McQuillan JJ, Rosen GD, Dean DC. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1). J Biol Chem. 1992; 267:16323–16329. PMID: 1379595.
Article34. Ledebur HC, Parks TP. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-kappa B site and p65 homodimers. J Biol Chem. 1995; 270:933–943. PMID: 7822333.35. Whelan J, Ghersa P, Hooft van, Gray J, Chandra G, Talabot F, DeLamarter JF. An NF kappa B-like factor is essential but not sufficient for cytokine induction of endothelial leukocyte adhesion molecule 1 (ELAM-1) gene transcription. Nucleic Acids Res. 1991; 19:2645–2653. PMID: 1710341.36. Kang JS, Park SK, Yang KH, Kim HM. Silymarin inhibits TNF-alpha-induced expression of adhesion molecules in human umbilical vein endothelial cells. FEBS Lett. 2003; 550:89–93. PMID: 12935892.37. Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996; 8:402–411. PMID: 8793994.
Article38. Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995; 270:7420–7426. PMID: 7535770.
Article39. Oh SJ, Jung SP, Han J, Kim S, Kim JS, Nam SJ, Lee JE, Kim JH. Silibinin inhibits TPA-induced cell migration and MMP-9 expression in thyroid and breast cancer cells. Oncol Rep. 2013; 29:1343–1348. PMID: 23353996.
Article40. Kim S, Choi JH, Lim HI, Lee SK, Kim WW, Kim JS, Kim JH, Choe JH, Yang JH, Nam SJ, Lee JE. Silibinin prevents TPAinduced MMP-9 expression and VEGF secretion by inactivation of the Raf/MEK/ERK pathway in MCF-7 human breast cancer cells. Phytomedicine. 2009; 16:573–580. PMID: 19181503.
Article41. Larsen L, Størling J, Darville M, Eizirik DL, Bonny C, Billestrup N, Mandrup-Poulsen T. Extracellular signal-regulated kinase is essential for interleukin-1-induced and nuclear factor kappaB-mediated gene expression in insulin-producing INS-1E cells. Diabetologia. 2005; 48:2582–2590. PMID: 16283237.42. Youn CK, Park SJ, Li MH, Lee MY, Lee KY, Cha MJ, Kim OH, You HJ, Chang IY, Yoon SP, Jeon YJ. Radicicol inhibits inos expression in cytokine-stimulated pancreatic beta cells. Korean J Physiol Pharmacol. 2013; 17:315–320. PMID: 23946691.
Article43. Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, Borisy GG. Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol. 2003; 160:409–421. PMID: 12566431.
Article44. Adams JC, Schwartz MA. Stimulation of fascin spikes by thrombospondin-1 is mediated by the GTPases Rac and Cdc42. J Cell Biol. 2000; 150:807–822. PMID: 10953005.
Article45. Hashimoto Y, Parsons M, Adams JC. Dual actin-bundling and protein kinase C-binding activities of fascin regulate carcinoma cell migration downstream of Rac and contribute to metastasis. Mol Biol Cell. 2007; 18:4591–4602. PMID: 17855511.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Silymarin Inhibits Cytokine-Stimulated Pancreatic Beta Cells by Blocking the ERK1/2 Pathway
- Anti-Inflammatory Effect of Mangostenone F in Lipopolysaccharide-Stimulated RAW264.7 Macrophages by Suppressing NF-kappaB and MAPK Activation
- The Regulatory Mechanism of Src Familly Kinase in Lipopolysaccharide (LPS) induced HF-kappaB Activation Pathway
- Aromadendrin Inhibits Lipopolysaccharide-Induced Nuclear Translocation of NF-kappaB and Phosphorylation of JNK in RAW 264.7 Macrophage Cells
- MyD88-BLT2-dependent cascade contributes to LPS-induced interleukin-6 production in mouse macrophage