Blood Res.
2019 Dec;54(4):253-261. 10.5045/br.2019.54.4.253.
Potential biomarkers and antagonists for fluoranthene-induced cellular toxicity of bone marrow derived mesenchymal stem cells
- Affiliations
-
- 1Department of Laboratory Medicine and Mitochondrial Research Laboratory, Chonnam National University Medical School, Chonnam National University Hwasun Hospital, Hwasun, Korea. mgshin@jnu.ac.kr
- 2Brain Korea 21 Plus Program, Chonnam National University Medical School, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 3College of Korean Medicine, Dongshin University, Naju, Korea. 98lani@gmail.com
- KMID: 2466590
- DOI: http://doi.org/10.5045/br.2019.54.4.253
Abstract
- BACKGROUND
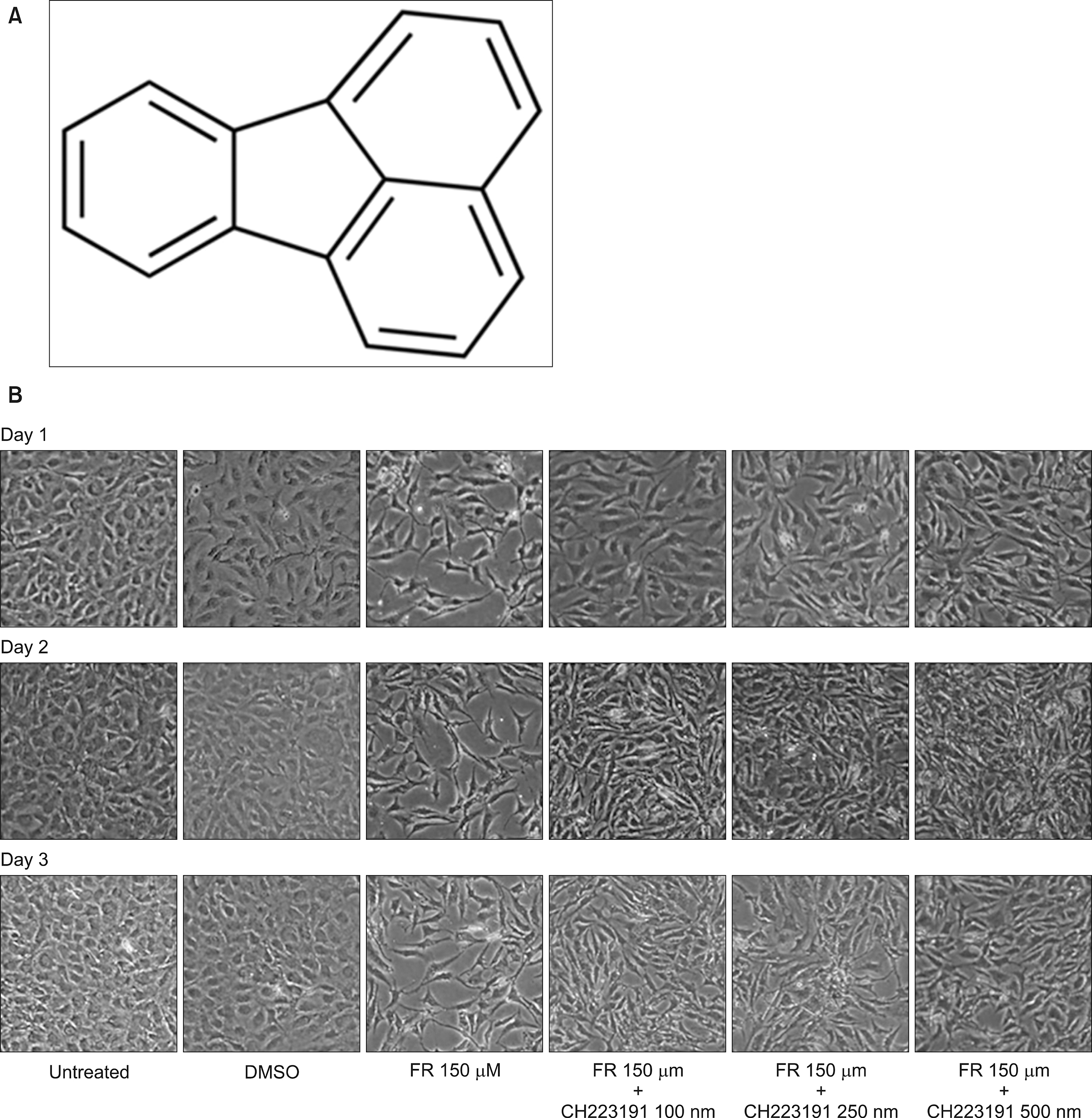
Fluoranthene (FR) is a common environmental pollutant that exists in a complex mixture with other polycyclic aromatic hydrocarbons (PAHs). We identified biomarkers for monitoring FR exposure and investigated the rescue effect of FR-induced cellular toxicity via aryl hydrocarbon receptor (AHR) antagonist activity in bone marrow derived mesenchymal stem cells (BM-MSCs).
METHODS
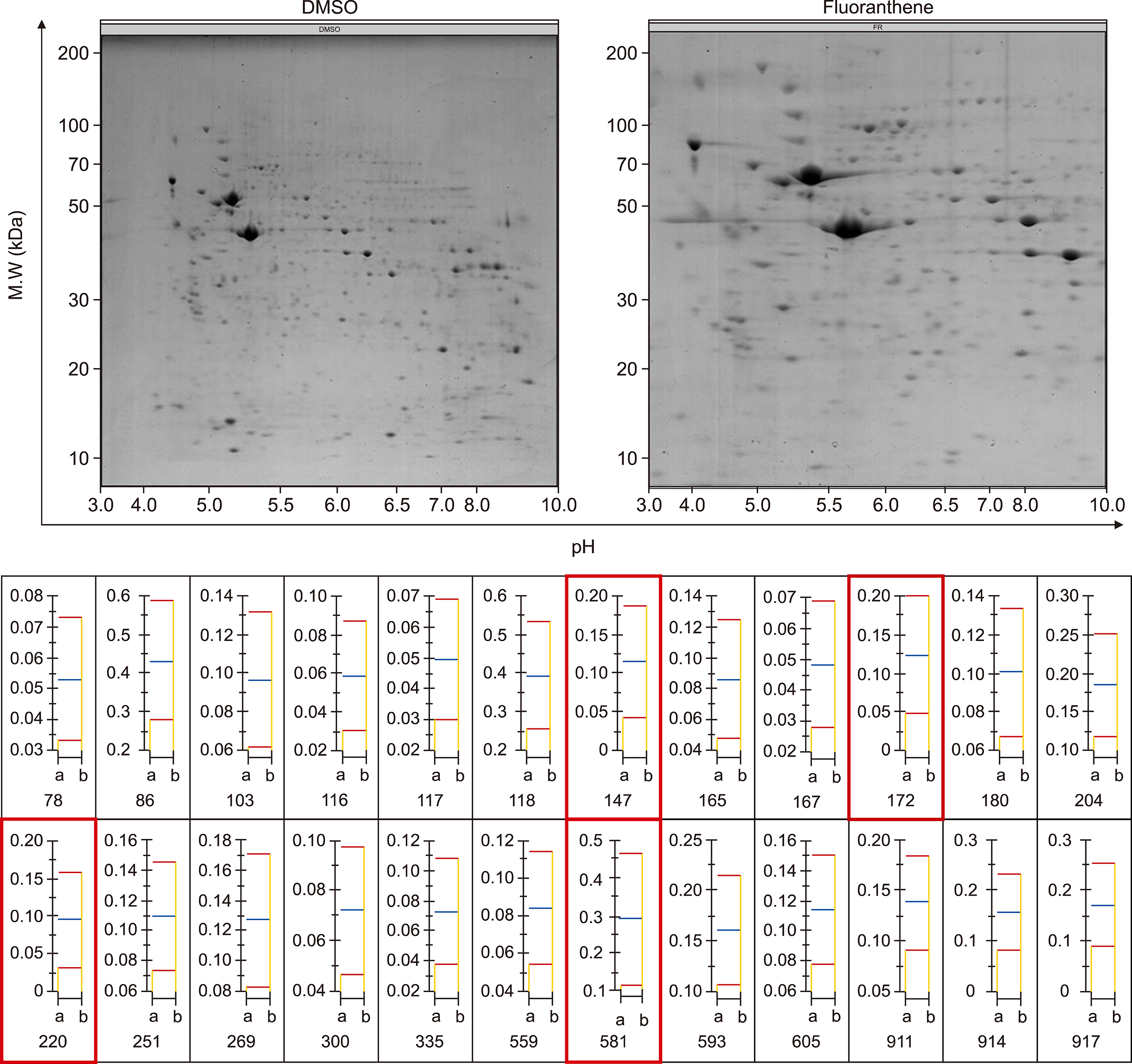
Morphological changes, viability, and rescue effects of an AHR antagonist (CH223191) were examined in BM-MSCs after exposure to FR. Cytotoxic effects were assayed using the tetrazolium-based colorimetric assay. Apoptosis was measured by annexin V and propidium iodide dye-based flowcytometry assay, mitochondrial membrane potential assay, and nuclear DNA fragmentation assay. Molecular signaling pathways of apoptosis and autophagy were investigated using immunoblotting. Proteomics were performed in order to reveal the spectra of cellular damage and identify biomarkers for FR exposure.
RESULTS
Exposing BM-MSCs to FR (ICâ‚…â‚€=50 µM) induced cell death and morphological changes, while the AHR antagonist showed rescue effects. Autophagy was activated and mitochondrial membrane potential was decreased. Proteomic analysis identified 48 deregulated proteins (26 upregulated and 22 downregulated). Among them, annexin A6, pyruvate kinase, UDP-glucose dehydrogenase, and phospholipase A2 could be potential biomarkers for FR exposure.
CONCLUSION
The exposure of BM-MSCs to FR induced remarkable alterations in cellular biology and the proteome, allowing for identification of novel biomarkers for FR exposure. Furthermore, AHR antagonists might be able to prevent cellular damage due to FR exposure.
MeSH Terms
-
Annexin A5
Annexin A6
Apoptosis
Autophagy
Biomarkers*
Bone Marrow*
Cell Death
DNA Fragmentation
Immunoblotting
Membrane Potential, Mitochondrial
Mesenchymal Stromal Cells*
Oxidoreductases
Phospholipases A2
Polycyclic Hydrocarbons, Aromatic
Propidium
Proteome
Proteomics
Pyruvate Kinase
Receptors, Aryl Hydrocarbon
Annexin A5
Annexin A6
Biomarkers
Oxidoreductases
Phospholipases A2
Polycyclic Hydrocarbons, Aromatic
Propidium
Proteome
Pyruvate Kinase
Receptors, Aryl Hydrocarbon
Figure
Reference
-
1. Raychoudhury SS, Kubinski D. Polycyclic aromatic hydrocarbon-induced cytotoxicity in cultured rat Sertoli cells involves differential apoptotic response. Environ Health Perspect. 2003; 111:33–38.
Article2. Cherng SH, Lin ST, Lee H. Modulatory effects of polycyclic aromatic hydrocarbons on the mutagenicity of 1-nitropyrene: a structure-activity relationship study. Mutat Res. 1996; 367:177–185.
Article3. Lewtas J, Walsh D, Williams R, Dobiás L. Air pollution exposure-DNA adduct dosimetry in humans and rodents: evidence for non-linearity at high doses. Mutat Res. 1997; 378:51–63.
Article4. Zedeck MS. Polycyclic aromatic hydrocarbons: a review. J Environ Pathol Toxicol. 1980; 3:537–567.5. Busbee DL, Norman JO, Ziprin RL. Comparative uptake, vascular transport, and cellular internalization of aflatoxin-B1 and benzo(a)pyrene. Arch Toxicol. 1990; 64:285–290.
Article6. Monteith DK, Novotny A, Michalopoulos G, Strom SC. Metabolism of benzo[a]pyrene in primary cultures of human hepatocytes: dose-response over a four-log range. Carcinogenesis. 1987; 8:983–988.
Article7. Kiefer F, Cumpelik O, Wiebel FJ. Metabolism and cytotoxicity of benzo(a)pyrene in the human lung tumour cell line NCI-H322. Xenobiotica. 1988; 18:747–755.
Article8. McKinney PA, Fear NT, Stockton D;. Parental occupation at periconception: findings from the United Kingdom Childhood Cancer Study. Occup Environ Med. 2003; 60:901–909.
Article9. Castro-Jiménez MÁ, Orozco-Vargas LC. Parental exposure to carcinogens and risk for childhood acute lymphoblastic leukemia, Colombia, 2000-2005. Prev Chronic Dis. 2011; 8:A106.10. Chang JS. Parental smoking and childhood leukemia. Methods Mol Biol. 2009; 472:103–137.
Article11. Milne E, Greenop KR, Scott RJ, et al. Parental prenatal smoking and risk of childhood acute lymphoblastic leukemia. Am J Epidemiol. 2012; 175:43–53.
Article12. Visser O, van Wijnen JH, van Leeuwen FE. Residential traffic density and cancer incidence in Amsterdam, 1989-1997. Cancer Causes Control. 2004; 15:331–339.
Article13. Pearson RL, Wachtel H, Ebi KL. Distance-weighted traffic density in proximity to a home is a risk factor for leukemia and other childhood cancers. J Air Waste Manag Assoc. 2000; 50:175–180.
Article14. Griesbaum K, Behr A, Biedenkapp D, et al. Hydrocarbons. In : Elvers B, editor. Ullmann's encyclopedia of industrial chemistry. 7th ed. Weinheim, Germany: Wiley-VCH;2011.15. Busby WF Jr, Goldman ME, Newberne PM, Wogan GN. Tumorigenicity of fluoranthene in a newborn mouse lung adenoma bioassay. Carcinogenesis. 1984; 5:1311–1316.
Article16. Zhu H, Li Y, Trush MA. Characterization of benzo[a]pyrene quinone-induced toxicity to primary cultured bone marrow stromal cells from DBA/2 mice: potential role of mitochondrial dysfunction. Toxicol Appl Pharmacol. 1995; 130:108–120.
Article17. Li N, Sioutas C, Cho A, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003; 111:455–460.
Article18. Xia T, Korge P, Weiss JN, et al. Quinones and aromatic chemical compounds in particulate matter induce mitochondrial dysfunction: implications for ultrafine particle toxicity. Environ Health Perspect. 2004; 112:1347–1358.
Article19. Ko CB, Kim SJ, Park C, et al. Benzo(a)pyrene-induced apoptotic death of mouse hepatoma Hepa1c1c7 cells via activation of intrinsic caspase cascade and mitochondrial dysfunction. Toxicology. 2004; 199:35–46.
Article20. Kim HY, Kim HR, Kang MG, et al. Profiling of biomarkers for the exposure of polycyclic aromatic hydrocarbons: lamin-A/C isoform 3, poly[ADP-ribose] polymerase 1, and mitochondria copy number are identified as universal biomarkers. Biomed Res Int. 2014; 2014:605135.
Article21. Gnecchi M, Melo LG. Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol. 2009; 482:281–294.
Article22. Xie J, Techritz S, Haebel S, et al. A two-dimensional electrophoretic map of human mitochondrial proteins from immortalized lymphoblastoid cell lines: a prerequisite to study mitochondrial disorders in patients. Proteomics. 2005; 5:2981–2999.
Article23. Cossarizza A, Franceschi C, Monti D, et al. Protective effect of N-acetylcysteine in tumor necrosis factor-alpha-induced apoptosis in U937 cells: the role of mitochondria. Exp Cell Res. 1995; 220:232–240.
Article24. Castedo M, Macho A, Zamzami N, et al. Mitochondrial perturbations define lymphocytes undergoing apoptotic depletion in vivo. Eur J Immunol. 1995; 25:3277–3284.
Article25. Marchetti P, Susin SA, Decaudin D, et al. Apoptosis-associated derangement of mitochondrial function in cells lacking mitochondrial DNA. Cancer Res. 1996; 56:2033–2038.26. Zhao B, Degroot DE, Hayashi A, He G, Denison MS. CH223191 is a ligand-selective antagonist of the Ah (Dioxin) receptor. Toxicol Sci. 2010; 117:393–403.
Article27. Kim SH, Henry EC, Kim DK, et al. Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol. 2006; 69:1871–1878.
Article28. Poot M, Zhang YZ, Krämer JA, et al. Analysis of mitochondrial morphology and function with novel fixable fluorescent stains. J Histochem Cytochem. 1996; 44:1363–1372.
Article29. Cornely R, Rentero C, Enrich C, Grewal T, Gaus K. Annexin A6 is an organizer of membrane microdomains to regulate receptor localization and signalling. IUBMB Life. 2011; 63:1009–1017.
Article30. Atsumi G, Tajima M, Hadano A, Nakatani Y, Murakami M, Kudo I. Fas-induced arachidonic acid release is mediated by Ca2+-independent phospholipase A2 but not cytosolic phospholipase A2, which undergoes proteolytic inactivation. J Biol Chem. 1998; 273:13870–13877.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Expression of surface markers and myogenic potential of rat bone marrow- and adipose-derived stem cells: a comparative study
- Stem Cells and Niemann Pick Disease
- Bone marrow-derived stem cells contribute to regeneration of the endometrium