Anat Cell Biol.
2013 Jun;46(2):113-121. 10.5115/acb.2013.46.2.113.
Expression of surface markers and myogenic potential of rat bone marrow- and adipose-derived stem cells: a comparative study
- Affiliations
-
- 1Cellular and Molecular Research Center, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. bayati-v@ajums.ac.ir
- 2Department of Anatomical Science, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
- 3Department of Anatomy and Cell Biology, Gilan University of Medical Sciences, Rasht, Iran.
- 4Department of Anatomical Sciences, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran.
- KMID: 2263103
- DOI: http://doi.org/10.5115/acb.2013.46.2.113
Abstract
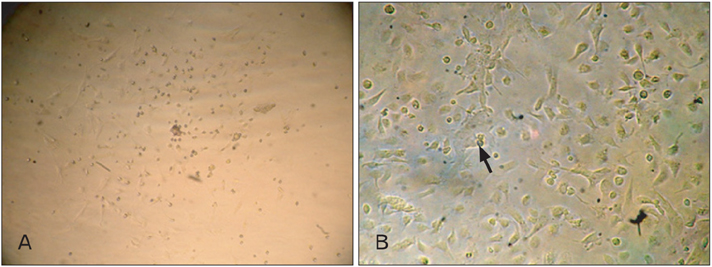
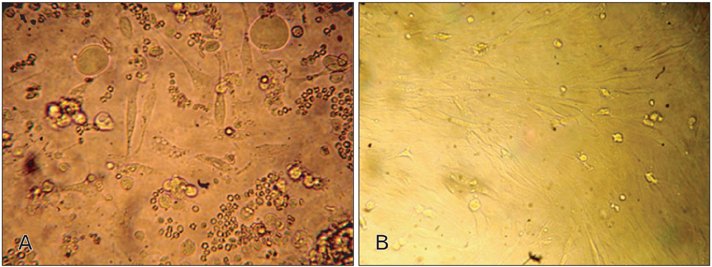
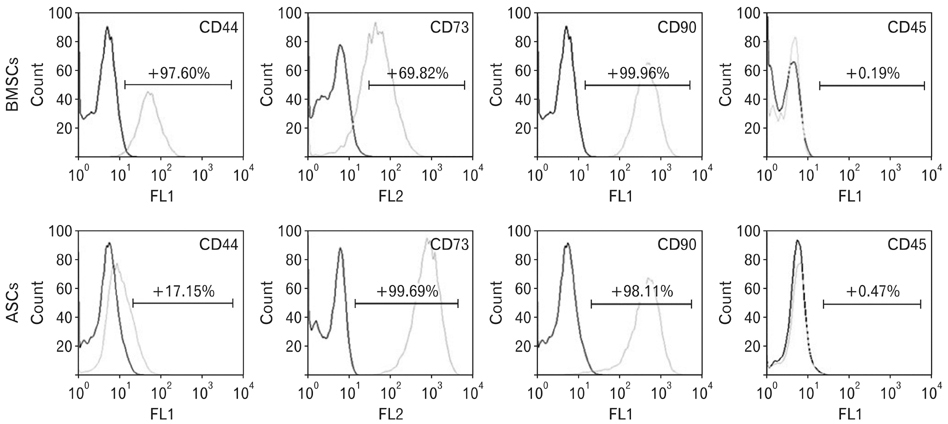
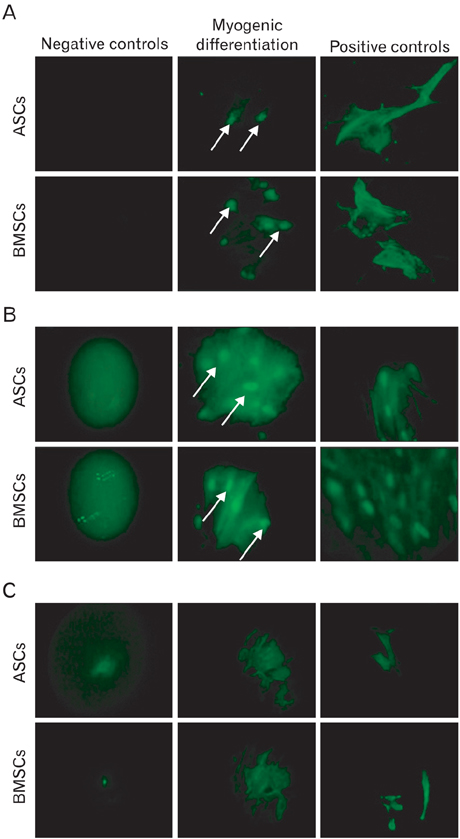
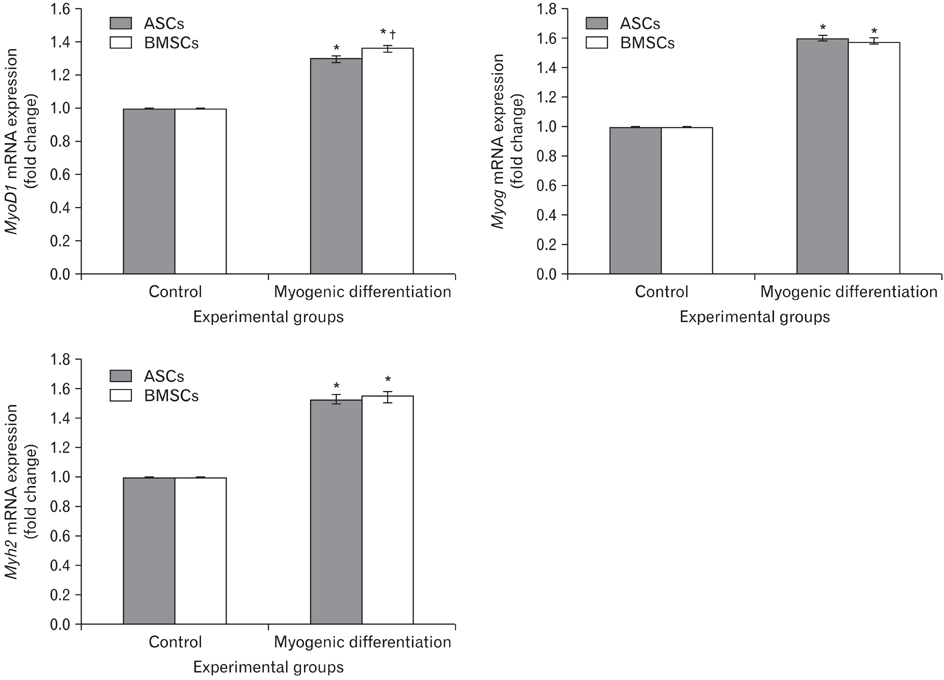
- In recent years, examination and comparison of the biological characteristics of bone marrow- and adipose-derived mesenchymal stem cells (MSCs) from various perspectives have come into the focus of stem cell research, as these cells should be well characterized in order to utilize them in future cellular therapies. Therefore, in the present study, surface protein markers and the skeletal myogenic differentiation potential of rat bone marrow- and adipose-derived MSCs were examined. The expression of CD44, CD45, CD73, and CD90 on bone marrow- and adipose-derived MSCs was characterized using flow cytometry. Subsequently, the stem cells were differentiated into myogenic lineages, and the expression of the skeletal myogenic markers MyoD1, Myog, and Myh2 was studied in cells using real time polymerase chain reaction and immunofluorescence. Our results reveal that the pattern of CD marker expression differs between these 2 types of MSCs to some extent, whereas no significant difference was observed with respect to their myogenic differentiation potential. Therefore, we concluded that despite the differences observed in the biological features of these 2 types of MSCs, their myogenic potential appears to be similar, and that adipose-derived stem cells may be useful in skeletal muscle tissue engineering, due to their easy isolation and capacity for rapid expansion in a short time span.
Keyword
MeSH Terms
Figure
Reference
-
1. Ahn SM, Simpson R, Lee B. Genomics and proteomics in stem cell research: the road ahead. Anat Cell Biol. 2010. 43:1–14.2. Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol. 2012. 5:19.3. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007. 25:2739–2749.4. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007. 100:1249–1260.5. Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008. 45:115–120.6. Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006. 24:150–154.7. Rasmussen JG, Frobert O, Holst-Hansen C, Kastrup J, Baandrup U, Zachar V, Fink T, Simonsen U. Comparison of Human Adipose-Derived Stem Cells and Bone Marrow-Derived Stem Cells in a Myocardial Infarction Model. Cell Transplant. 2012. 12. 4. [Epub] http://dx.doi.org/10.3727/096368912X659871.8. Kishimoto S, Ishihara M, Mori Y, Takikawa M, Hattori H, Nakamura S, Sato T. Effective expansion of human adipose-derived stromal cells and bone marrow-derived mesenchymal stem cells cultured on a fragmin/protamine nanoparticles-coated substratum with human platelet-rich plasma. J Tissue Eng Regen Med. 2012. 03. 31. [Epub] http://dx.doi.org/10.1002/term.1488.9. Reich CM, Raabe O, Wenisch S, Bridger PS, Kramer M, Arnhold S. Isolation, culture and chondrogenic differentiation of canine adipose tissue- and bone marrow-derived mesenchymal stem cells: a comparative study. Vet Res Commun. 2012. 36:139–148.10. Zimmerlin L, Donnenberg VS, Rubin JP, Donnenberg AD. Mesenchymal markers on human adipose stem/progenitor cells. Cytometry A. 2013. 83:134–140.11. Qi Z, Zhang Y, Liu L, Guo X, Qin J, Cui G. Mesenchymal stem cells derived from different origins have unique sensitivities to different chemotherapeutic agents. Cell Biol Int. 2012. 06. 14. [Epub] http://dx.doi.org/10.1002/term.1488.12. Mantovani C, Raimondo S, Haneef MS, Geuna S, Terenghi G, Shawcross SG, Wiberg M. Morphological, molecular and functional differences of adult bone marrow- and adipose-derived stem cells isolated from rats of different ages. Exp Cell Res. 2012. 318:2034–2048.13. Zhu X, Shi W, Tai W, Liu F. The comparition of biological characteristics and multilineage differentiation of bone marrow and adipose derived Mesenchymal stem cells. Cell Tissue Res. 2012. 350:277–287.14. Torensma R, Prins HJ, Schrama E, Verwiel ET, Martens AC, Roelofs H, Jansen BJ. The impact of cell source, culture methodology, culture location, and individual donors on gene expression profiles of bone marrow-derived and adipose-derived stromal cells. Stem Cells Dev. 2013. 22:1086–1096.15. Puetzer J, Williams J, Gillies A, Bernacki S, Loboa EG. The effects of cyclic hydrostatic pressure on chondrogenesis and viability of human adipose- and bone marrow-derived mesenchymal stem cells in three-dimensional agarose constructs. Tissue Eng Part A. 2013. 19:299–306.16. Di Rocco G, Iachininoto MG, Tritarelli A, Straino S, Zacheo A, Germani A, Crea F, Capogrossi MC. Myogenic potential of adipose-tissue-derived cells. J Cell Sci. 2006. 119:2945–2952.17. Belema Bedada F, Technau A, Ebelt H, Schulze M, Braun T. Activation of myogenic differentiation pathways in adult bone marrow-derived stem cells. Mol Cell Biol. 2005. 25:9509–9519.18. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006. 8:315–317.19. Semedo P, Correa-Costa M, Antonio Cenedeze M, Maria Avancini Costa Malheiros D, Antonia dos Reis M, Shimizu MH, Seguro AC, Pacheco-Silva A, Saraiva Camara NO. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009. 27:3063–3073.20. Semedo P, Palasio CG, Oliveira CD, Feitoza CQ, Gonçalves GM, Cenedeze MA, Wang PM, Teixeira VP, Reis MA, Pacheco-Silva A, Câmara NO. Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int Immunopharmacol. 2009. 9:677–682.21. Li B, Zeng Q, Wang H, Shao S, Mao X, Zhang F, Li S, Guo Z. Adipose tissue stromal cells transplantation in rats of acute myocardial infarction. Coron Artery Dis. 2007. 18:221–227.22. Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004. 103:1662–1668.23. Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004. 32:414–425.24. Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003. 89:1235–1249.25. Gronthos S, Simmons PJ, Graves SE, Robey PG. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone. 2001. 28:174–181.26. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002. 13:4279–4295.27. Docheva D, Haasters F, Schieker M. Mesenchymal stem cells and their cell surface receptors. Curr Rheumatol Rev. 2008. 4:155–160.28. Meligy FY, Shigemura K, Behnsawy HM, Fujisawa M, Kawabata M, Shirakawa T. The efficiency of in vitro isolation and myogenic differentiation of MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue. In Vitro Cell Dev Biol Anim. 2012. 48:203–215.29. de la Garza-Rodea AS, van der Velde-van Dijke I, Boersma H, Gonçalves MA, van Bekkum DW, de Vries AA, Knaän-Shanzer S. Myogenic properties of human mesenchymal stem cells derived from three different sources. Cell Transplant. 2012. 21:153–173.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- A study on the osteogenic differentiation of adipose-derived adult stem cell
- Chondrogenesis of Mesenchymal Stem Cell Derived from Canine Adipose Tissue
- Investigating Cell Surface Markers on Normal Hematopoietic Stem Cells in Three Different Niche Conditions
- Synergistic Effect of Hydrogen and 5-Aza on Myogenic Differentiation through the p38 MAPK Signaling Pathway in Adipose-Derived Mesenchymal Stem Cells