Int J Stem Cells.
2023 Feb;16(1):78-92. 10.15283/ijsc21238.
Synergistic Effect of Hydrogen and 5-Aza on Myogenic Differentiation through the p38 MAPK Signaling Pathway in Adipose-Derived Mesenchymal Stem Cells
- Affiliations
-
- 1Department of Sports Medicine, Northern Jiangsu People’s Hospital, Clinical Medical College, Yangzhou University, Yangzhou, China
- 2Department of Sports Medicine, Northern Jiangsu People’s Hospital, Dalian Medical University, Dalian, China
- KMID: 2539623
- DOI: http://doi.org/10.15283/ijsc21238
Abstract
- Background and Objectives
This study aims to clarify the systems underlying regulation and regulatory roles of hydrogen combined with 5-Aza in the myogenic differentiation of adipose mesenchymal stem cells (ADSCs).
Methods and Results
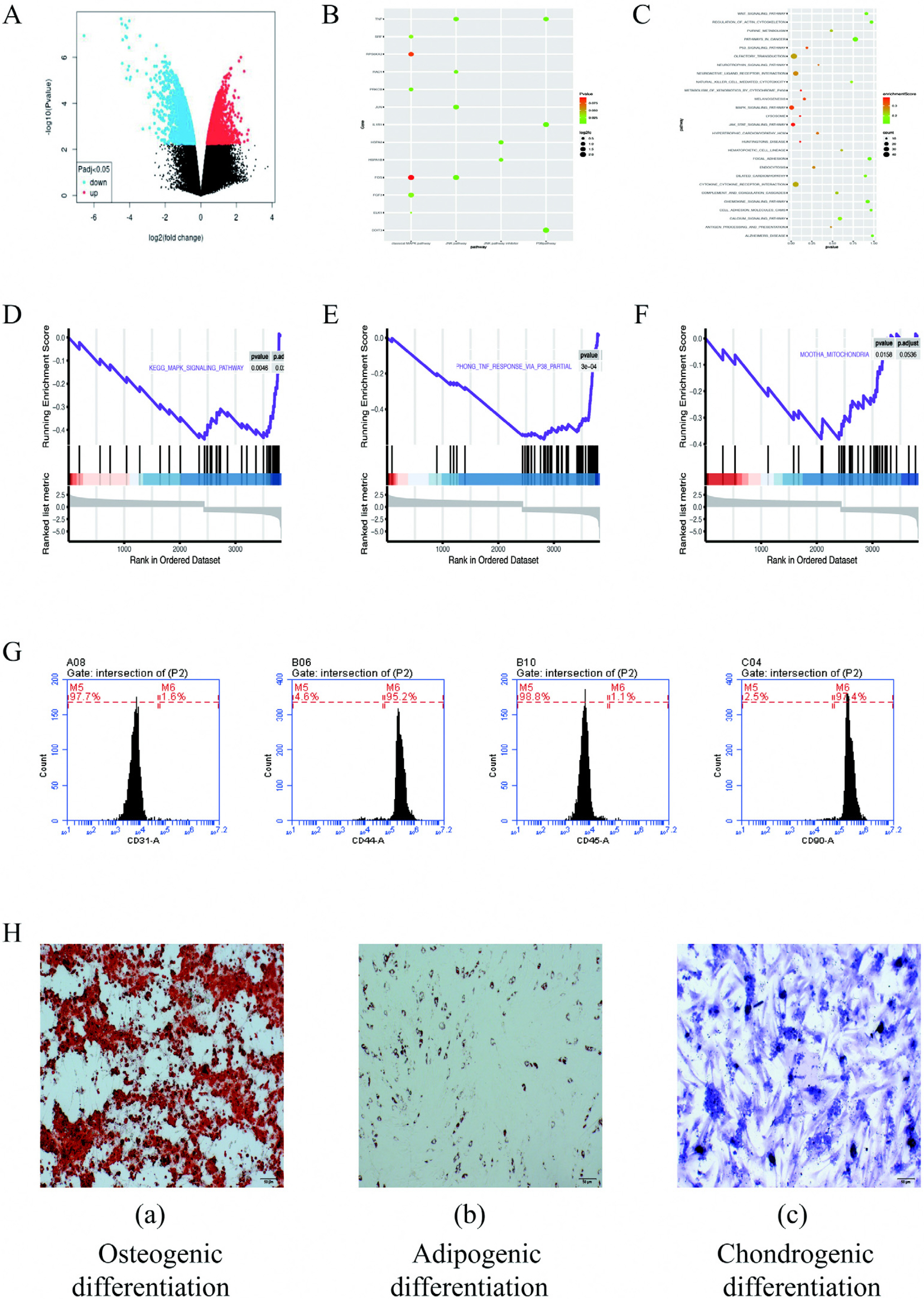
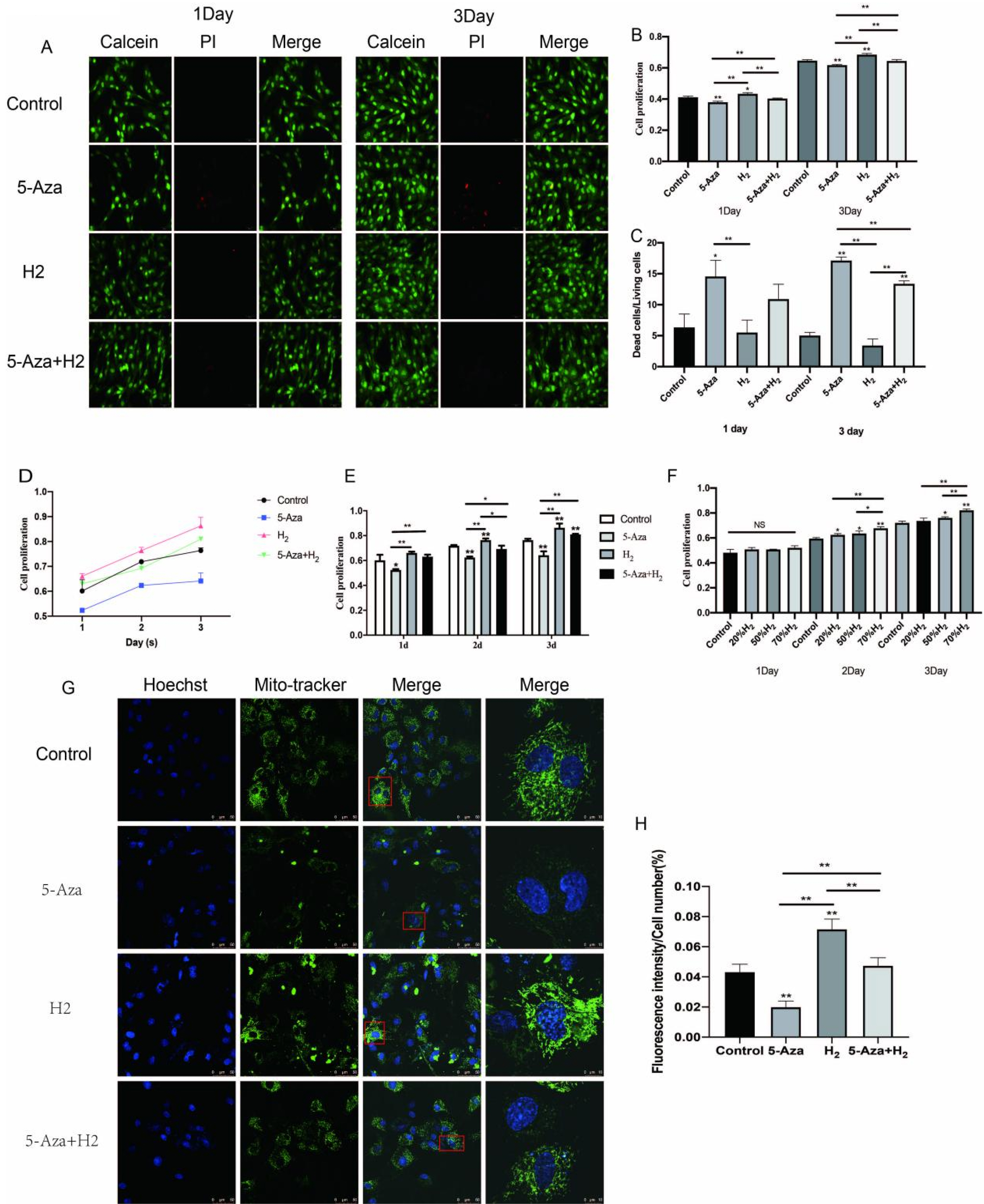
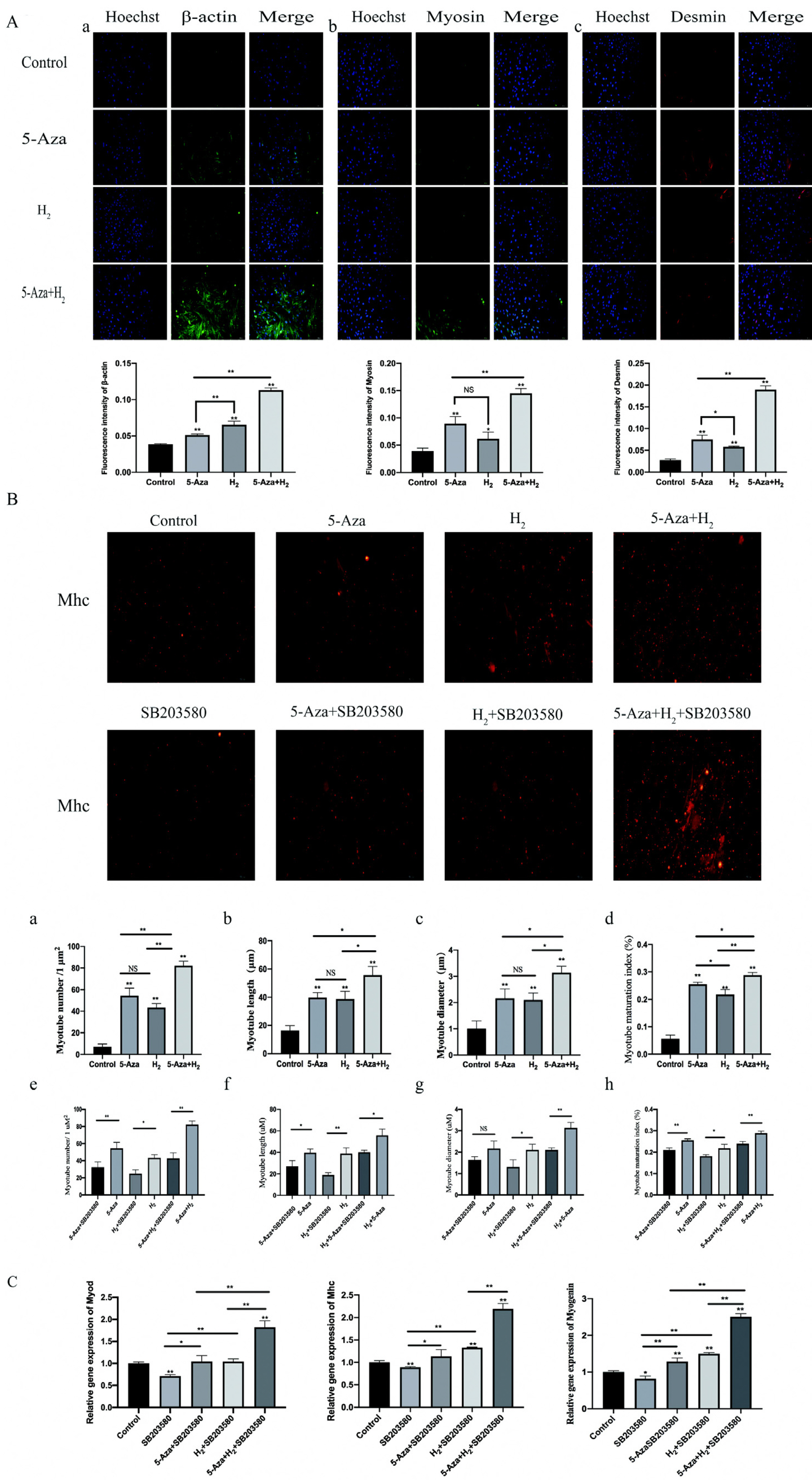
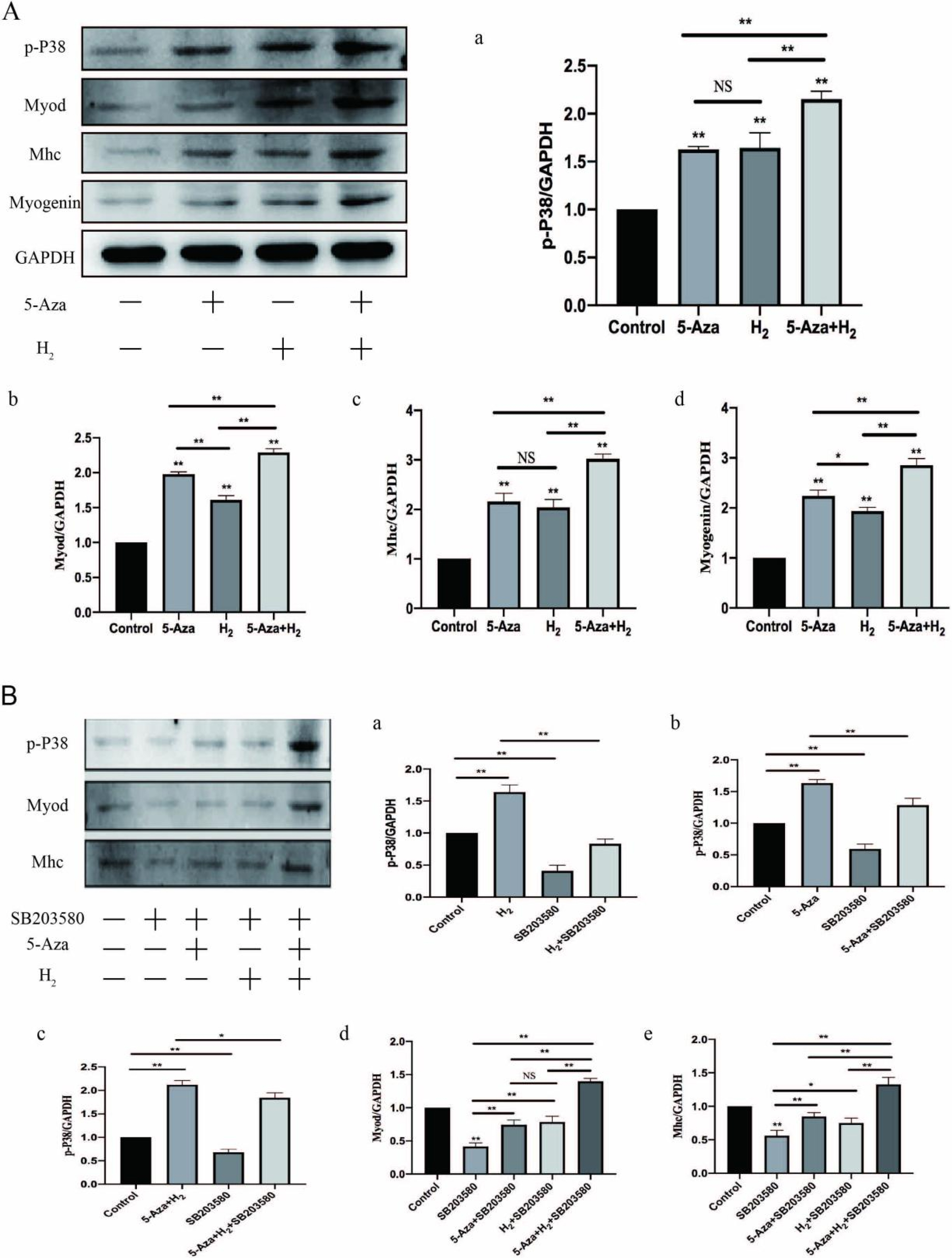
In this study, ADSCs acted as an in vitro myogenic differentiating mode. First, the Alamar blue Staining and mitochondrial tracer technique were used to verify whether hydrogen combined with 5-Aza could promote cell proliferation. In addition, this study assessed myogenic differentiating markers (e.g., Myogenin, Mhc and Myod protein expressions) based on the Western blotting assay, analysis on cellular morphological characteristics (e.g., Myotube number, length, diameter and maturation index), RT-PCR (Myod, Myogenin and Mhc mRNA expression) and Immunofluorescence analysis (Desmin, Myosin and β-actin protein expression). Finally, to verify the mechanism of myogenic differentiation of hydrogen-bound 5-Aza, we performed bioinformatics analysis and Western blot to detect the expression of p-P38 protein. Hydrogen combined with 5-Aza significantly enhanced the proliferation and myogenic differentiation of ADSCs in vitro by increasing the number of single-cell mitochondria and upregulating the expression of myogenic biomarkers such as Myod, Mhc and myotube formation. The expressions of p-P38 was up-regulated by hydrogen combined with 5-Aza. The differentiating ability was suppressed when the cells were cultivated in combination with SB203580 (p38 MAPK signal pathway inhibitor).
Conclusions
Hydrogen alleviates the cytotoxicity of 5-Aza and synergistically promotes the myogenic differentiation capacity of adipose stem cells via the p38 MAPK pathway. Thus, the mentioned results present insights into myogenic differentiation and are likely to generate one potential alternative strategy for skeletal muscle related diseases.
Keyword
Figure
Reference
-
References
1. Abmayr SM, Pavlath GK. 2012; Myoblast fusion: lessons from flies and mice. Development. 139:641–656. DOI: 10.1242/dev.068353. PMID: 22274696. PMCID: PMC3265056.2. Greising SM, Warren GL, Southern WM, Nichenko AS, Qualls AE, Corona BT, Call JA. 2018; Early rehabilitation for volumetric muscle loss injury augments endogenous regenerative aspects of muscle strength and oxidative capacity. BMC Musculoskelet Disord. 19:173. DOI: 10.1186/s12891-018-2095-6. PMID: 29843673. PMCID: PMC5975473.3. Aguilar CA, Greising SM, Watts A, Goldman SM, Peragallo C, Zook C, Larouche J, Corona BT. 2018; Multiscale analysis of a regenerative therapy for treatment of volumetric muscle loss injury. Cell Death Discov. 4:33. Erratum in: Cell Death Discov 2018;5:16. DOI: 10.1038/s41420-018-0080-3. PMID: 30062061. PMCID: PMC6056478.4. Valencia Mora M, Ruiz Ibán MA, Díaz Heredia J, Barco Laakso R, Cuéllar R, García Arranz M. 2015; Stem cell therapy in the management of shoulder rotator cuff disorders. World J Stem Cells. 7:691–699. DOI: 10.4252/wjsc.v7.i4.691. PMID: 26029341. PMCID: PMC4444610.5. Hernigou P, Flouzat Lachaniette CH, Delambre J, Zilber S, Duffiet P, Chevallier N, Rouard H. 2014; Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 38:1811–1818. DOI: 10.1007/s00264-014-2391-1. PMID: 24913770.6. Gimble JM, Katz AJ, Bunnell BA. 2007; Adipose-derived stem cells for regenerative medicine. Circ Res. 100:1249–1260. DOI: 10.1161/01.RES.0000265074.83288.09. PMID: 17495232. PMCID: PMC5679280.7. Grottkau BE, Lin Y. 2013; Osteogenesis of adipose-derived stem cells. Bone Res. 1:133–145. DOI: 10.4248/BR201302003. PMID: 26273498. PMCID: PMC4472098.8. Dai R, Wang Z, Samanipour R, Koo KI, Kim K. 2016; Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int. 2016:6737345. DOI: 10.1155/2016/6737345. PMID: 27057174. PMCID: PMC4761677.9. Xie X, Wang Y, Zhao C, Guo S, Liu S, Jia W, Tuan RS, Zhang C. 2012; Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials. 33:7008–7018. DOI: 10.1016/j.biomaterials.2012.06.058. PMID: 22818985.10. Liu Y, Yan X, Sun Z, Chen B, Han Q, Li J, Zhao RC. 2007; Flk-1+ adipose-derived mesenchymal stem cells differentiate into skeletal muscle satellite cells and ameliorate muscular dystrophy in mdx mice. Stem Cells Dev. 16:695–706. DOI: 10.1089/scd.2006.0118. PMID: 17999592.11. Abdanipour A, Tiraihi T, Delshad A. 2011; Trans-differentiation of the adipose tissue-derived stem cells into neuron-like cells expressing neurotrophins by selegiline. Iran Biomed J. 15:113–121. DOI: 10.6091/ibj.1011.2012. PMID: 22395135. PMCID: PMC3614245.12. Mizuno H, Zuk PA, Zhu M, Lorenz HP, Benhaim P, Hedrick MH. 2002; Myogenic differentiation by human processed lipoaspirate cells. Plast Reconstr Surg. 109:199–209. discussion 210–211. DOI: 10.1097/00006534-200201000-00031. PMID: 11786812.13. McClure MJ, Garg K, Simpson DG, Ryan JJ, Sell SA, Bowlin GL, Ericksen JJ. 2016; The influence of platelet-rich plasma on myogenic differentiation. J Tissue Eng Regen Med. 10:E239–E249. DOI: 10.1002/term.1755. PMID: 23868863.14. Zhao P, Jin Z, Chen Q, Yang T, Chen D, Meng J, Lu X, Gu Z, He Q. 2018; Local generation of hydrogen for enhanced photothermal therapy. Nat Commun. 9:4241. DOI: 10.1038/s41467-018-06630-2. PMID: 30315173. PMCID: PMC6185976. PMID: f9bc03749b6242b09102f738b42273b3.15. Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. 2007; Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 13:688–694. DOI: 10.1038/nm1577. PMID: 17486089.16. Ono H, Nishijima Y, Adachi N, Sakamoto M, Kudo Y, Nakazawa J, Kaneko K, Nakao A. 2012; Hydrogen(H2) treatment for acute erythymatous skin diseases. A report of 4 patients with safety data and a non-controlled feasibility study with H2 concentration measurement on two volunteers. Med Gas Res. 2:14. DOI: 10.1186/2045-9912-2-14. PMID: 22607973. PMCID: PMC3407032.17. Guan P, Sun ZM, Luo LF, Zhou J, Yang S, Zhao YS, Yu FY, An JR, Wang N, Ji ES. 2019; Hydrogen protects against chronic intermittent hypoxia induced renal dysfunction by promoting autophagy and alleviating apoptosis. Life Sci. 225:46–54. DOI: 10.1016/j.lfs.2019.04.005. PMID: 30951745.18. Li J, Hong Z, Liu H, Zhou J, Cui L, Yuan S, Chu X, Yu P. 2016; Hydrogen-rich saline promotes the recovery of renal function after ischemia/reperfusion injury in rats via anti-apoptosis and anti-inflammation. Front Pharmacol. 7:106. DOI: 10.3389/fphar.2016.00106. PMID: 27148060. PMCID: PMC4840252.19. Miyazaki N, Yamaguchi O, Nomiya M, Aikawa K, Kimura J. 2016; Preventive effect of hydrogen water on the development of detrusor overactivity in a rat model of bladder outlet obstruction. J Urol. 195:780–787. DOI: 10.1016/j.juro.2015.10.117. PMID: 26518110.20. Quan-Jun Y, Yan H, Yong-Long H, Li-Li W, Jie L, Jin-Lu H, Jin L, Peng-Guo C, Run G, Cheng G. 2017; Selumetinib attenuates skeletal muscle wasting in murine cachexia model through ERK inhibition and AKT activation. Mol Cancer Ther. 16:334–343. DOI: 10.1158/1535-7163.MCT-16-0324. PMID: 27599525.21. Suzuki A, Minamide R, Iwata J. 2018; WNT/β-catenin signaling plays a crucial role in myoblast fusion through regulation of nephrin expression during development. Development. 145:dev168351. DOI: 10.1242/dev.168351. PMID: 30389854. PMCID: PMC6288386.22. Low S, Barnes JL, Zammit PS, Beauchamp JR. 2018; Delta-like 4 activates Notch 3 to regulate self-renewal in skeletal muscle stem cells. Stem Cells. 36:458–466. DOI: 10.1002/stem.2757. PMID: 29230914.23. Böhm A, Hoffmann C, Irmler M, Schneeweiss P, Schnauder G, Sailer C, Schmid V, Hudemann J, Machann J, Schick F, Beckers J, Hrabě de Angelis M, Staiger H, Fritsche A, Stefan N, Nieß AM, Häring HU, Weigert C. 2016; TGF-β contributes to impaired exercise response by suppression of mitochondrial key regulators in skeletal muscle. Diabetes. 65:2849–2861. DOI: 10.2337/db15-1723. PMID: 27358493.24. Yi C, Liu D, Fong CC, Zhang J, Yang M. 2010; Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano. 4:6439–6448. DOI: 10.1021/nn101373r. PMID: 21028783.25. Lassar AB. 2009; The p38 MAPK family, a pushmi-pullyu of skeletal muscle differentiation. J Cell Biol. 187:941–943. DOI: 10.1083/jcb.200911123. PMID: 20026653. PMCID: PMC2806286.26. Jones NC, Tyner KJ, Nibarger L, Stanley HM, Cornelison DD, Fedorov YV, Olwin BB. 2005; The p38alpha/beta MAPK functions as a molecular switch to activate the quiescent satellite cell. J Cell Biol. 169:105–116. DOI: 10.1083/jcb.200408066. PMID: 15824134. PMCID: PMC2171902.27. Wakitani S, Saito T, Caplan AI. 1995; Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 18:1417–1426. DOI: 10.1002/mus.880181212. PMID: 7477065.28. Yang M, Dong Y, He Q, Zhu P, Zhuang Q, Shen J, Zhang X, Zhao M. 2020; Hydrogen: a novel option in human disease treatment. Oxid Med Cell Longev. 2020:8384742. DOI: 10.1155/2020/8384742. PMID: 32963703. PMCID: PMC7495244.29. Ohta S. 2014; Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacol Ther. 144:1–11. DOI: 10.1016/j.pharmthera.2014.04.006. PMID: 24769081.30. Huang CS, Kawamura T, Toyoda Y, Nakao A. 2010; Recent advances in hydrogen research as a therapeutic medical gas. Free Radic Res. 44:971–982. DOI: 10.3109/10715762.2010.500328. PMID: 20815764.31. Smith GCS, Bouwmeester TM, Lam PH. 2017; Knotless double-row SutureBridge rotator cuff repairs have improved self-reinforcement compared with double-row SutureBridge repairs with tied medial knots: a biomechanical study using an ovine model. J Shoulder Elbow Surg. 26:2206–2212. DOI: 10.1016/j.jse.2017.06.045. PMID: 28935379.32. Kim KC, Shin HD, Cha SM, Park JY. 2014; Comparisons of retear patterns for 3 arthroscopic rotator cuff repair methods. Am J Sports Med. 42:558–565. DOI: 10.1177/0363546514521577. PMID: 24585674.33. Killian ML, Cavinatto LM, Ward SR, Havlioglu N, Thomopoulos S, Galatz LM. 2015; Chronic degeneration leads to poor healing of repaired massive rotator cuff tears in rats. Am J Sports Med. 43:2401–2410. DOI: 10.1177/0363546515596408. PMID: 26297522. PMCID: PMC4750378.34. Papa L, Djedaini M, Hoffman R. 2019; Mitochondrial role in stemness and differentiation of hematopoietic stem cells. Stem Cells Int. 2019:4067162. DOI: 10.1155/2019/4067162. PMID: 30881461. PMCID: PMC6381553.35. Zhang H, Menzies KJ, Auwerx J. 2018; The role of mitochondria in stem cell fate and aging. Development. 145:dev143420. DOI: 10.1242/dev.143420. PMID: 29654217. PMCID: PMC5964648.36. Ciciliot S, Schiaffino S. 2010; Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Curr Pharm Des. 16:906–914. Erratum in: Curr Pharm Des 2015;21:4657. DOI: 10.2174/138161210790883453. PMID: 20041823.37. Yu X, Zhang Y, Li T, Ma Z, Jia H, Chen Q, Zhao Y, Zhai L, Zhong R, Li C, Zou X, Meng J, Chen AK, Puri PL, Chen M, Zhu D. 2017; Long non-coding RNA Linc-RAM enhances myogenic differentiation by interacting with MyoD. Nat Commun. 8:14016. DOI: 10.1038/ncomms14016. PMID: 28091529. PMCID: PMC5241866. PMID: 1c57eaf4e6a84d45965f21b426993e40.38. Paulin D, Li Z. 2004; Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp Cell Res. 301:1–7. DOI: 10.1016/j.yexcr.2004.08.004. PMID: 15501438.39. Singh K, Dilworth FJ. 2013; Differential modulation of cell cycle progression distinguishes members of the myogenic regulatory factor family of transcription factors. FEBS J. 280:3991–4003. DOI: 10.1111/febs.12188. PMID: 23419170.40. Yokoyama S, Asahara H. 2011; The myogenic transcriptional network. Cell Mol Life Sci. 68:1843–1849. DOI: 10.1007/s00018-011-0629-2. PMID: 21318263. PMCID: PMC3092062.41. Le Moal E, Pialoux V, Juban G, Groussard C, Zouhal H, Chazaud B, Mounier R. 2017; Redox control of skeletal muscle regeneration. Antioxid Redox Signal. 27:276–310. DOI: 10.1089/ars.2016.6782. PMID: 28027662. PMCID: PMC5685069.42. Ji LL, Kang C, Zhang Y. 2016; Exercise-induced hormesis and skeletal muscle health. Free Radic Biol Med. 98:113–122. DOI: 10.1016/j.freeradbiomed.2016.02.025. PMID: 26916558.43. Mason SA, Morrison D, McConell GK, Wadley GD. 2016; Muscle redox signalling pathways in exercise. Role of antioxidants. Free Radic Biol Med. 98:29–45. DOI: 10.1016/j.freeradbiomed.2016.02.022. PMID: 26912034.44. Gibbons MC, Singh A, Engler AJ, Ward SR. 2018; The role of mechanobiology in progression of rotator cuff muscle atrophy and degeneration. J Orthop Res. 36:546–556. DOI: 10.1002/jor.23662. PMCID: PMC5788743. PMID: 28755470.45. Oh JH, Chung SW, Kim SH, Chung JY, Kim JY. 2014; 2013 neer award: effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J Shoulder Elbow Surg. 23:445–455. DOI: 10.1016/j.jse.2013.07.054. PMID: 24129058.46. Pas HIMFL, Moen MH, Haisma HJ, Winters M. 2017; No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med. 51:996–1002. DOI: 10.1136/bjsports-2016-096794. PMID: 28077355.47. Osborne H, Anderson L, Burt P, Young M, Gerrard D. 2016; Australasian College of Sports Physicians-position statement: the place of mesenchymal stem/stromal cell therapies in sport and exercise medicine. Br J Sports Med. 50:1237–1244. DOI: 10.1136/bjsports-2015-095711. PMID: 26701927.48. Saito T, Sadoshima J. 2015; Molecular mechanisms of mitochondrial autophagy/mitophagy in the heart. Circ Res. 116:1477–1490. DOI: 10.1161/CIRCRESAHA.116.303790. PMID: 25858070. PMCID: PMC4419704.49. Murakami Y, Ito M, Ohsawa I. 2017; Molecular hydrogen protects against oxidative stress-induced SH-SY5Y neuroblastoma cell death through the process of mitohormesis. PLoS One. 12:e0176992. DOI: 10.1371/journal.pone.0176992. PMID: 28467497. PMCID: PMC5415102.50. Qi R, Liu H, Wang Q, Wang J, Yang F, Long D, Huang J. 2017; Expressions and regulatory effects of P38/ERK/JNK Mapks in the adipogenic trans-differentiation of C2C12 myoblasts. Cell Physiol Biochem. 44:2467–2475. DOI: 10.1159/000486169. PMID: 29268268.51. Bernet JD, Doles JD, Hall JK, Kelly Tanaka K, Carter TA, Olwin BB. 2014; p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat Med. 20:265–271. DOI: 10.1038/nm.3465. PMID: 24531379. PMCID: PMC4070883.52. Brien P, Pugazhendhi D, Woodhouse S, Oxley D, Pell JM. 2013; p38α MAPK regulates adult muscle stem cell fate by restricting progenitor proliferation during postnatal growth and repair. Stem Cells. 31:1597–1610. DOI: 10.1002/stem.1399. PMID: 23592450.53. Cuenda A, Sanz-Ezquerro JJ. 2017; p38γ and p38δ: from spectators to key physiological players. Trends Biochem Sci. 42:431–442. DOI: 10.1016/j.tibs.2017.02.008. PMID: 28473179.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- IL6 Receptor Facilitates Adipogenesis Differentiation of Human Mesenchymal Stem Cells through Activating P38 Pathway
- Nectandrin A Enhances the BMP-Induced Osteoblastic Differentiation and Mineralization by Activation of p38 MAPK-Smad Signaling Pathway
- Cannabidiol Promotes Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells in the Inflammatory Microenvironment via the CB2-dependent p38 MAPK Signaling Pathway
- Expression of surface markers and myogenic potential of rat bone marrow- and adipose-derived stem cells: a comparative study
- Thromboxane A2 modulates migration, proliferation, and differentiation of adipose tissue-derived mesenchymal stem cells