Korean J Physiol Pharmacol.
2013 Oct;17(5):447-453. 10.4196/kjpp.2013.17.5.447.
Nectandrin A Enhances the BMP-Induced Osteoblastic Differentiation and Mineralization by Activation of p38 MAPK-Smad Signaling Pathway
- Affiliations
-
- 1Department of Pharmacology and Clinical Pharmacy, College of Pharmacy, Kyung Hee University, Seoul 130-701, Korea. suchung@khu.ac.kr
- KMID: 1500241
- DOI: http://doi.org/10.4196/kjpp.2013.17.5.447
Abstract
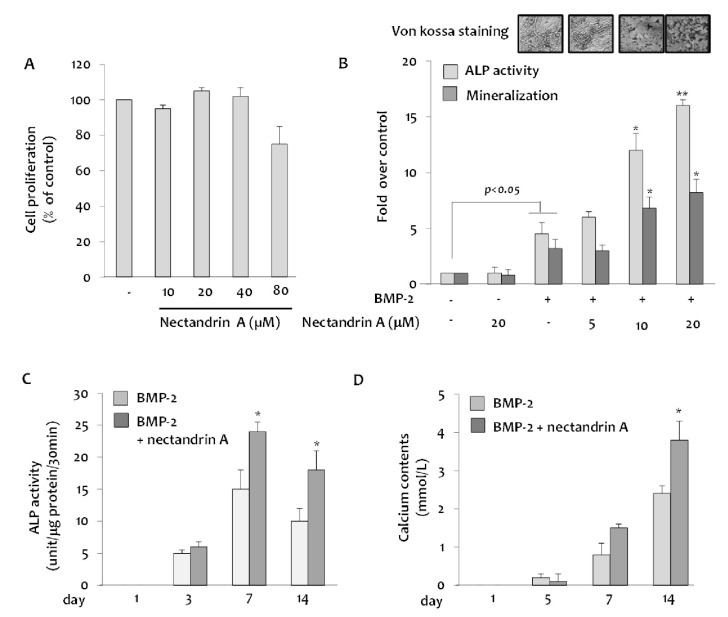
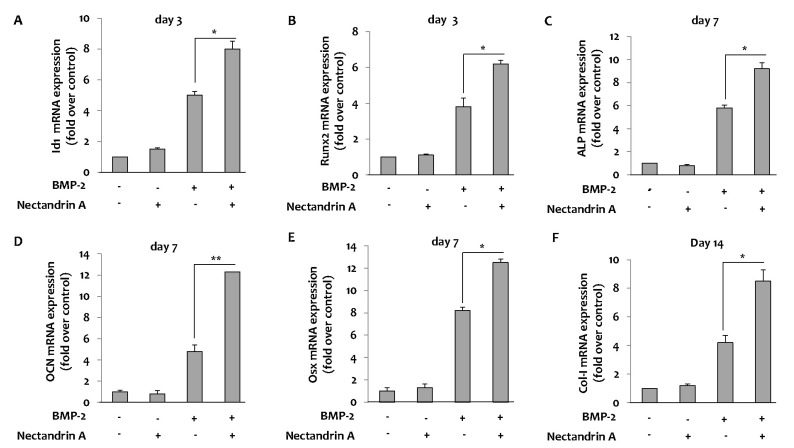
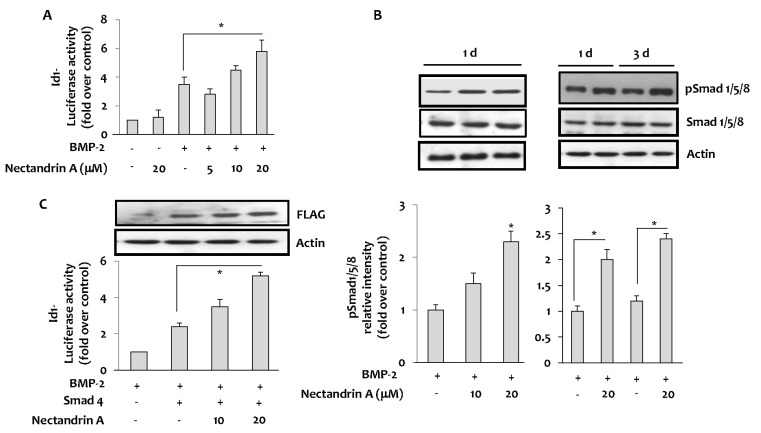
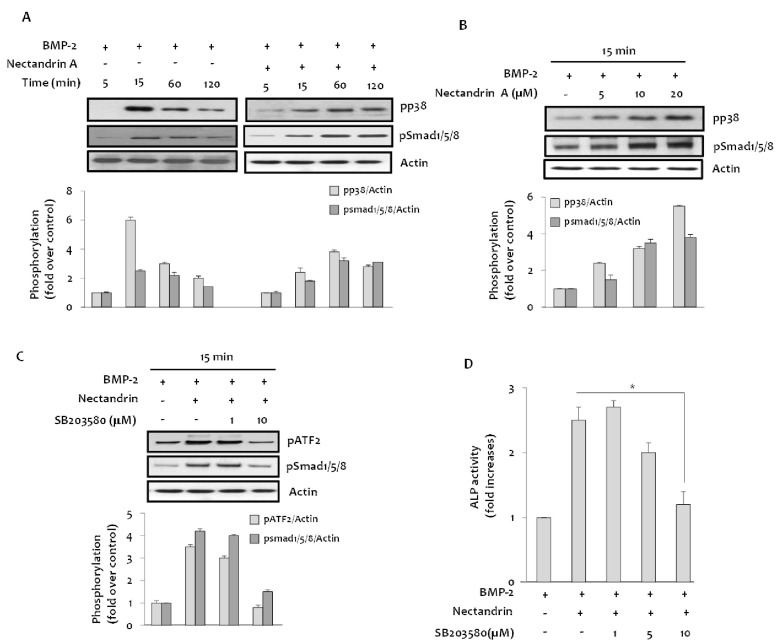
- Osteoblastic activity of nectandrin A was examined in C2C12 cells. Nectandrin A enhances the BMP-induced osteoblastic differentiation and mineralization, manifested by the up-regulation of differentiation markers (alkaline phosphatase and osteogenic genes) and increased calcium contents. In C2C12 cells co-transfected with expression vector encoding Smad4 and Id1-Luc reporter, nectandrin A increased Id1 luciferase activity in a concentration-dependent manner, when compared to that in BMP-2 treated cells, indicating that Smad signaling pathway is associated with nectandrin A-enhanced osteoblastic differentiation in C2C12 cells. In addition, nectandrin A activated p38 mitogen-activated protein kinase (MAPK) in time- and concentration-dependent manners, and phosphorylated form of pSmad1/5/8 and alkaline phosphatase activity were both decreased when the cells were pretreated with SB203580, a p38 MAPK inhibitor, suggesting that p38 MAPK might be an upstream kinase for Smad signaling pathway. Taken together, nectandrin A enhances the BMP-induced osteoblastic differentiation and mineralization of C2C12 cells via activation of p38 MAPK-Smad signaling pathway, and it has a therapeutic potential for osteoporosis by promoting bone formation.
Keyword
MeSH Terms
-
Alkaline Phosphatase
Antigens, Differentiation
Calcium
Imidazoles
Luciferases
Osteoblasts*
Osteogenesis
Osteoporosis
p38 Mitogen-Activated Protein Kinases
Phosphotransferases
Protein Kinases
Pyridines
Up-Regulation
Alkaline Phosphatase
Antigens, Differentiation
Calcium
Imidazoles
Luciferases
Phosphotransferases
Protein Kinases
Pyridines
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
1. Miao D, He B, Jiang Y, Kobayashi T, Sorocéanu MA, Zhao J, Su H, Tong X, Amizuka N, Gupta A, Genant HK, Kronenberg HM, Goltzman D, Karaplis AC. Osteoblast-derived PTHrP is a potent endogenous bone anabolic agent that modifies the therapeutic efficacy of administered PTH 1-34. J Clin Invest. 2005; 115:2402–2411. PMID: 16138191.
Article2. Genant HK, Baylink DJ, Gallagher JC. Estrogens in the prevention of osteoporosis in postmenopausal women. Am J Obstet Gynecol. 1989; 161:1842–1846. PMID: 2690636.
Article3. Reid IR. Pharmacotherapy of osteoporosis in postmenopausal women: focus on safety. Expert Opin Drug Saf. 2002; 1:93–107. PMID: 12904164.
Article4. Turner CH. Homeostatic control of bone structure: an application of feedback theory. Bone. 1991; 12:203–217. PMID: 1910962.
Article5. Ma CJ, Kim YC, Sung SH. Compounds with neuroprotective activity from the medicinal plant Machilus thunbergii. J Enzyme Inhib Med Chem. 2009; 24:1117–1121. PMID: 19555186.6. Lee MK, Yang H, Ma CJ, Kim YC. Stimulatory activity of lignans from Machilus thunbergii on osteoblast differentiation. Biol Pharm Bull. 2007; 30:814–817. PMID: 17409528.
Article7. Park BY, Min BS, Kwon OK, Oh SR, Ahn KS, Kim TJ, Kim DY, Bae K, Lee HK. Increase of caspase-3 activity by lignans from Machilus thunbergii in HL-60 cells. Biol Pharm Bull. 2004; 27:1305–1307. PMID: 15305043.
Article8. Sowa H, Kaji H, Yamaguchi T, Sugimoto T, Chihara K. Smad3 promotes alkaline phosphatase activity and mineralization of osteoblastic MC3T3-E1 cells. J Bone Miner Res. 2002; 17:1190–1199. PMID: 12096832.
Article9. Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. 2000; 289:1501–1504. PMID: 10968779.
Article10. Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010; 147:35–51. PMID: 19762341.
Article11. Chen G, Deng C, Li YP. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012; 8:272–288. PMID: 22298955.
Article12. Vukicevic S, Luyten FP, Reddi AH. Stimulation of the expression of osteogenic and chondrogenic phenotypes in vitro by osteogenin. Proc Natl Acad Sci U S A. 1989; 86:8793–8797. PMID: 2554330.13. Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994; 127:1755–1766. PMID: 7798324.14. Yamaguchi A, Katagiri T, Ikeda T, Wozney JM, Rosen V, Wang EA, Kahn AJ, Suda T, Yoshiki S. Recombinant human bone morphogenetic protein-2 stimulates osteoblastic maturation and inhibits myogenic differentiation in vitro. J Cell Biol. 1991; 113:681–687. PMID: 1849907.
Article15. Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem. 2002; 277:4883–4891. PMID: 11729207.
Article16. Peng Y, Kang Q, Luo Q, Jiang W, Si W, Liu BA, Luu HH, Park JK, Li X, Luo J, Montag AG, Haydon RC, He TC. Inhibitor of DNA binding/differentiation helix-loop-helix proteins mediate bone morphogenetic protein-induced osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004; 279:32941–32949. PMID: 15161906.
Article17. Maeda Y, Tsuji K, Nifuji A, Noda M. Inhibitory helix-loop-helix transcription factors Id1/Id3 promote bone formation in vivo. J Cell Biochem. 2004; 93:337–344. PMID: 15368360.18. Moldes M, Lasnier F, Fève B, Pairault J, Djian P. Id3 prevents differentiation of preadipose cells. Mol Cell Biol. 1997; 17:1796–1804. PMID: 9121427.
Article19. Itoh S, Itoh F, Goumans MJ, Ten Dijke P. Signaling of transforming growth factor-beta family members through Smad proteins. Eur J Biochem. 2000; 267:6954–6967. PMID: 11106403.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Zinc upregulates bone-specific transcription factor Runx2 expression via BMP-2 signaling and Smad-1 phosphorylation in osteoblasts
- Recombinant human bone morphogenic protein-2 Induces the Differentiation and Mineralization of Osteoblastic Cells Under Hypoxic Conditions via Activation of Protein Kinase D and p38 Mitogen-Activated Protein Kinase Signaling Pathways
- BMP‑6 Attenuates Oxygen and Glucose Deprivation-Induced Apoptosis in Human Neural Stem Cells through Inhibiting p38 MAPK Signaling Pathway
- Gemigliptin Inhibits Interleukin-1β–Induced Endothelial-Mesenchymal Transition via Canonical-Bone Morphogenetic Protein Pathway
- Effect of BMP-7 on osteoblastic differentiation of rat periodontal ligament cells