Int J Stem Cells.
2022 May;15(2):144-154. 10.15283/ijsc21093.
BMP‑6 Attenuates Oxygen and Glucose Deprivation-Induced Apoptosis in Human Neural Stem Cells through Inhibiting p38 MAPK Signaling Pathway
- Affiliations
-
- 1Department of Obstetrics and Gynecology, The First Affiliated Hospital of Xi’an Medical University, Xi’an, China
- KMID: 2529786
- DOI: http://doi.org/10.15283/ijsc21093
Abstract
- Background and Objectives
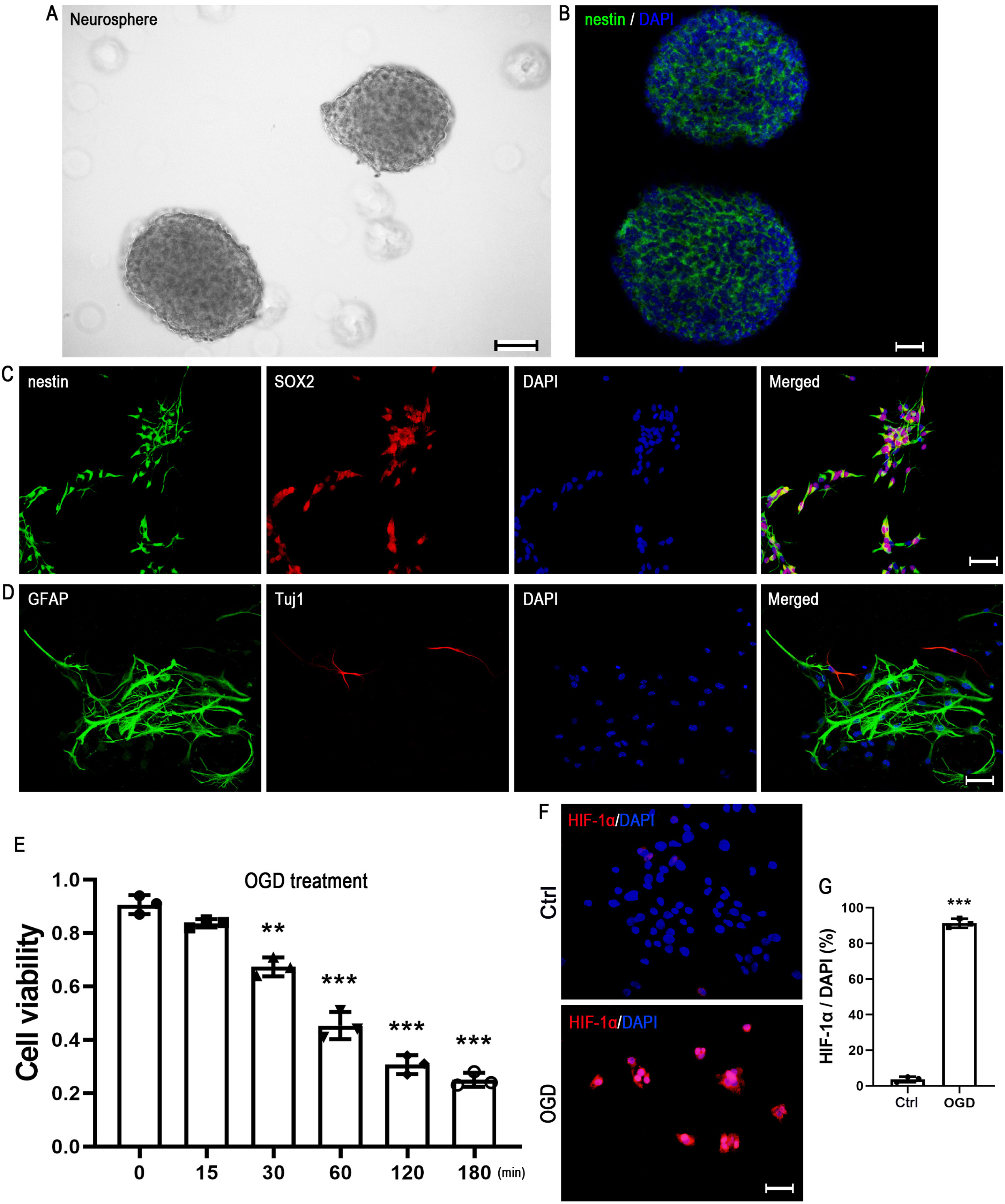
Neural stem cells (NSCs) remain in the mammalian brain throughout life and provide a novel therapeutic strategy for central nervous system (CNS) injury. Bone morphogenetic protein-6 (BMP-6) had shown a protective effect in different types of cells. However, the role of BMP-6 in NSCs is largely unclear. The present study was aimed to investigate whether BMP-6 could protect human NSCs (hNSCs) against the oxygen and glucose deprivation (OGD)-induced cell death.
Methods and Results
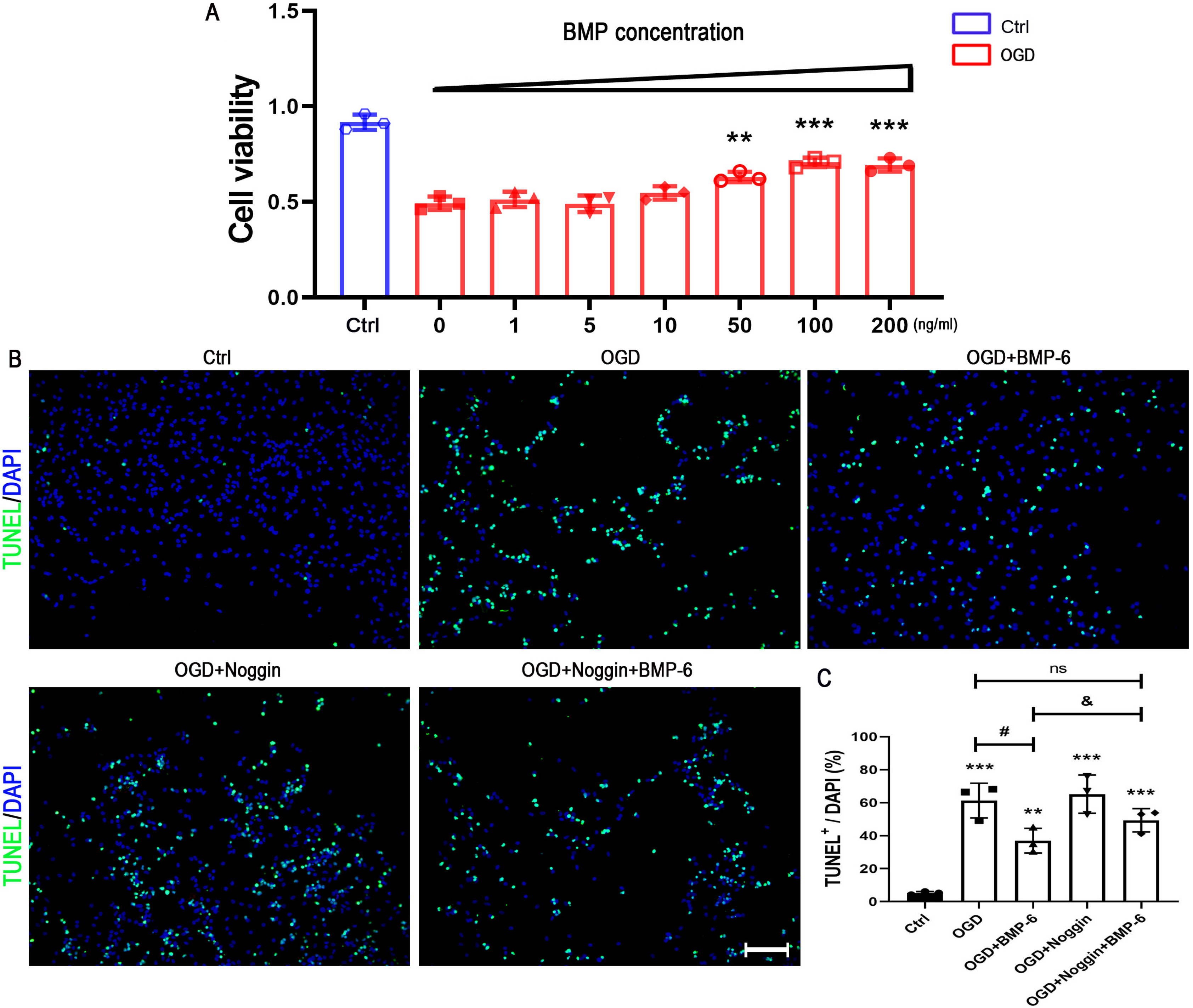
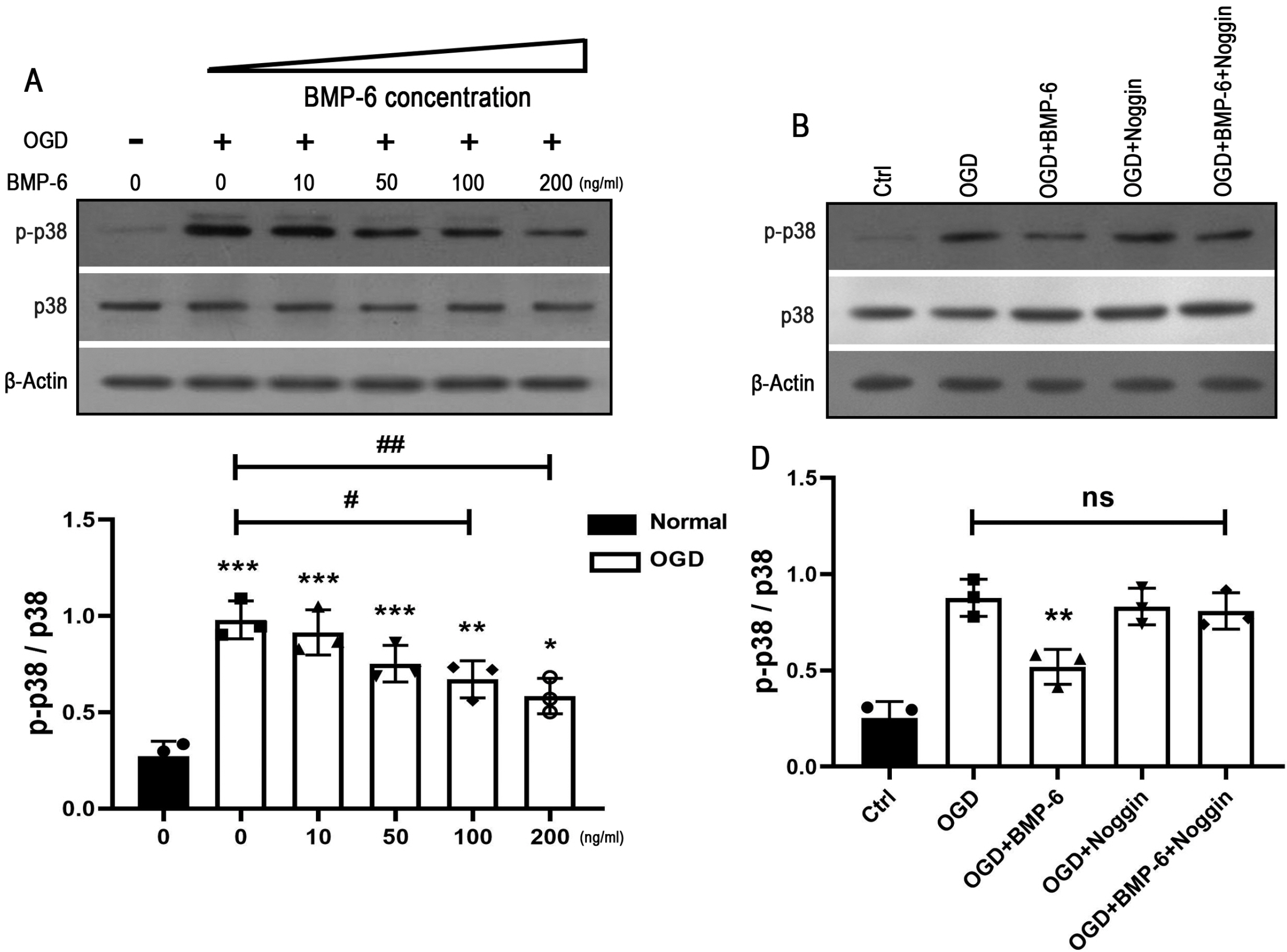
Upon challenge with OGD treatment, cell viability was significantly decreased in a time-dependent manner, as indicated by the CCK-8 assay. BMP-6 could attenuate the OGD-induced cell injury in a dose-dependent manner and decrease the number of TUNEL-positive cells. Moreover, BMP-6 markedly weakened the OGD-induced alterations in the expression of procaspase-8/9/3 and reversed the expression of cleaved-caspase-3. Interestingly, noggin protein (the BMP-6 inhibitor) attenuated the neuroprotective effect of BMP-6 in cultured hNSCs. Furthermore, the p38 MAPK signaling pathway was activated by OGD treatment and BMP-6 markedly inhibited the phosphorylation of p38 in a concentration-dependent manner. Pretreatment with noggin abolished the effect of BMP-6 on p38 activation. SB239063, a selective p38 inhibitor, exerted similar effects with BMP-6 in protecting hNSCs against the OGD-induced apoptosis. These results indicated that blocking the phosphorylation of p38 might contribute to the neuroprotective effect of BMP-6 against the OGD-induced injury in hNSCs.
Conclusions
These findings suggested that BMP-6 might be a therapeutic target in the OGD-induced cell death, which provides a novel therapeutic strategy for enhancing host and graft NSCs survival in hypoxic-ischemic brain injury.
Keyword
Figure
Reference
-
References
1. Patching SG. 2017; Glucose transporters at the blood-brain barrier: function, regulation and gateways for drug delivery. Mol Neurobiol. 54:1046–1077. DOI: 10.1007/s12035-015-9672-6. PMID: 26801191.
Article2. Rajchgot T, Thomas SC, Wang JC, Ahmadi M, Balood M, Crosson T, Dias JP, Couture R, Claing A, Talbot S. 2019; Neurons and microglia; a sickly-sweet duo in diabetic pain neuropathy. Front Neurosci. 13:25. DOI: 10.3389/fnins.2019.00025. PMID: 30766472. PMCID: PMC6365454.
Article3. Tucker LD, Lu Y, Dong Y, Yang L, Li Y, Zhao N, Zhang Q. 2018; Photobiomodulation therapy attenuates hypoxic-ischemic injury in a neonatal rat model. J Mol Neurosci. 65:514–526. DOI: 10.1007/s12031-018-1121-3. PMID: 30032397. PMCID: PMC6109412.
Article4. Wechsler LR, Bates D, Stroemer P, Andrews-Zwilling YS, Aizman I. 2018; Cell therapy for chronic stroke. Stroke. 49:1066–1074. DOI: 10.1161/STROKEAHA.117.018290. PMID: 29669865.
Article5. Sekhon MS, Ainslie PN, Griesdale DE. 2017; Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a "two-hit" model. Crit Care. 21:90. DOI: 10.1186/s13054-017-1670-9. PMID: 28403909. PMCID: PMC5390465.
Article6. Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wise MP, Åneman A, Al-Subaie N, Boesgaard S, Bro-Jeppesen J, Brunetti I, Bugge JF, Hingston CD, Juffermans NP, Koopmans M, Køber L, Langørgen J, Lilja G, Møller JE, Rundgren M, Rylander C, Smid O, Werer C, Winkel P, Friberg H. 2013; Targeted temperature management at 33℃ versus 36℃ after cardiac arrest. N Engl J Med. 369:2197–2206. DOI: 10.1056/NEJMoa1310519. PMID: 24237006.
Article7. Obernier K, Alvarez-Buylla A. 2019; Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain. Development. 146:dev156059. DOI: 10.1242/dev.156059. PMID: 30777863. PMCID: PMC6398449.
Article8. Ming GL, Song H. 2011; Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 70:687–702. DOI: 10.1016/j.neuron.2011.05.001. PMID: 21609825. PMCID: PMC3106107.
Article9. Burda JE, Sofroniew MV. 2014; Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 81:229–248. DOI: 10.1016/j.neuron.2013.12.034. PMID: 24462092. PMCID: PMC3984950.
Article10. Janowski M, Wagner DC, Boltze J. 2015; Stem cell-based tissue replacement after stroke: factual necessity or notorious fiction? Stroke. 46:2354–2363. DOI: 10.1161/STROKEAHA.114.007803. PMID: 26106118. PMCID: PMC4519410.11. Huang L, Zhang L. 2019; Neural stem cell therapies and hypoxic-ischemic brain injury. Prog Neurobiol. 173:1–17. DOI: 10.1016/j.pneurobio.2018.05.004. PMID: 29758244. PMCID: PMC6249121.
Article12. Hankey GJ. 2017; Stroke. Lancet. 389:641–654. DOI: 10.1016/S0140-6736(16)30962-X. PMID: 27637676.
Article13. Choe Y, Pleasure SJ, Mira H. 2015; Control of adult neurogenesis by short-range morphogenic-signaling molecules. Cold Spring Harb Perspect Biol. 8:a018887. DOI: 10.1101/cshperspect.a018887. PMID: 26637286. PMCID: PMC4772099.
Article14. Feng Y, Hu Y. 2018; Bone morphogenetic protein 9 serves a protective role in response to ischemic‑reperfusion in the brain by promoting ERK activation. Mol Med Rep. 17:2845–2852. DOI: 10.3892/mmr.2017.8253. PMID: 29257291. PMCID: PMC5783498.
Article15. Dizon ML, Maa T, Kessler JA. 2011; The bone morphogenetic protein antagonist noggin protects white matter after perinatal hypoxia-ischemia. Neurobiol Dis. 42:318–326. DOI: 10.1016/j.nbd.2011.01.023. PMID: 21310236. PMCID: PMC3079774.
Article16. Lee IH, Huang SS, Chuang CY, Liao KH, Chang LH, Chuang CC, Su YS, Lin HJ, Hsieh JY, Su SH, Lee OK, Kuo HC. 2017; Delayed epidural transplantation of human induced pluripotent stem cell-derived neural progenitors enhances functional recovery after stroke. Sci Rep. 7:1943. DOI: 10.1038/s41598-017-02137-w. PMID: 28512358. PMCID: PMC5434043. PMID: 237fb55871804c0987eccb30e40fc186.
Article17. Vukicevic S, Grgurevic L. 2009; BMP-6 and mesenchymal stem cell differentiation. Cytokine Growth Factor Rev. 20:441–448. DOI: 10.1016/j.cytogfr.2009.10.020. PMID: 19900832.
Article18. Jung Y, Song J, Shiozawa Y, Wang J, Wang Z, Williams B, Havens A, Schneider A, Ge C, Franceschi RT, McCauley LK, Krebsbach PH, Taichman RS. 2008; Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche. Stem Cells. 26:2042–2051. DOI: 10.1634/stemcells.2008-0149. PMID: 18499897. PMCID: PMC3513687.
Article19. Koike N, Kassai Y, Kouta Y, Miwa H, Konishi M, Itoh N. 2007; Brorin, a novel secreted bone morphogenetic protein antagonist, promotes neurogenesis in mouse neural precursor cells. J Biol Chem. 282:15843–15850. DOI: 10.1074/jbc.M701570200. PMID: 17400546.
Article20. Barneda-Zahonero B, Miñano-Molina A, Badiola N, Fadó R, Xifró X, Saura CA, Rodríguez-Alvarez J. 2009; Bone morphogenetic protein-6 promotes cerebellar granule neurons survival by activation of the MEK/ERK/CREB pathway. Mol Biol Cell. 20:5051–5063. DOI: 10.1091/mbc.e09-05-0424. PMID: 19846661. PMCID: PMC2793283.
Article21. Chen L, Liu M, Luan Y, Liu Y, Zhang Z, Ma B, Liu X, Liu Y. 2018; BMP‑6 protects retinal pigment epithelial cells from oxidative stress-induced injury by inhibiting the MAPK signaling pathways. Int J Mol Med. 42:1096–1105. DOI: 10.3892/ijmm.2018.3675. PMID: 29767257.
Article22. Lories RJ, Derese I, Ceuppens JL, Luyten FP. 2003; Bone morphogenetic proteins 2 and 6, expressed in arthritic synovium, are regulated by proinflammatory cytokines and differentially modulate fibroblast-like synoviocyte apoptosis. Arthritis Rheum. 48:2807–2818. DOI: 10.1002/art.11389. PMID: 14558086.
Article23. Uchiyama Y, Koike M, Shibata M. 2008; Autophagic neuron death in neonatal brain ischemia/hypoxia. Autophagy. 4:404–408. DOI: 10.4161/auto.5598. PMID: 18212531.
Article24. Hagberg H, David Edwards A, Groenendaal F. 2016; Perinatal brain damage: The term infant. Neurobiol Dis. 92(Pt A):102–112. DOI: 10.1016/j.nbd.2015.09.011. PMID: 26409031. PMCID: PMC4915441.
Article25. Salazar VS, Gamer LW, Rosen V. 2016; BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol. 12:203–221. DOI: 10.1038/nrendo.2016.12. PMID: 26893264.
Article26. Carreira AC, Lojudice FH, Halcsik E, Navarro RD, Sogayar MC, Granjeiro JM. 2014; Bone morphogenetic proteins: facts, challenges, and future perspectives. J Dent Res. 93:335–345. DOI: 10.1177/0022034513518561. PMID: 24389809.27. Miyazono K, Kamiya Y, Morikawa M. 2010; Bone morphogenetic protein receptors and signal transduction. J Biochem. 147:35–51. DOI: 10.1093/jb/mvp148. PMID: 19762341.
Article28. Tsai MJ, Weng CF, Shyue SK, Liou DY, Chen CH, Chiu CW, Yang TH, Pan HA, Liao RI, Kuo HS, Huang MC, Huang WC, Hoffer BJ, Cheng H. 2007; Dual effect of adenovirus-mediated transfer of BMP7 in mixed neuron-glial cultures: neuroprotection and cellular differentiation. J Neurosci Res. 85:2950–2959. DOI: 10.1002/jnr.21395. PMID: 17628501.
Article29. Chang CF, Morales M, Chou J, Chen HL, Hoffer B, Wang Y. 2002; Bone morphogenetic proteins are involved in fetal kidney tissue transplantation-induced neuroprotection in stroke rats. Neuropharmacology. 43:418–426. DOI: 10.1016/S0028-3908(02)00092-8. PMID: 12243771.
Article30. Bellenchi GC, Volpicelli F, Piscopo V, Perrone-Capano C, di Porzio U. 2013; Adult neural stem cells: an endogenous tool to repair brain injury? J Neurochem. 124:159–167. DOI: 10.1111/jnc.12084. PMID: 23134340.
Article31. Qu Q, Shi Y. 2009; Neural stem cells in the developing and adult brains. J Cell Physiol. 221:5–9. DOI: 10.1002/jcp.21862. PMID: 19562676. PMCID: PMC2869301.
Article32. Bond AM, Ming GL, Song H. 2015; Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell. 17:385–395. DOI: 10.1016/j.stem.2015.09.003. PMID: 26431181. PMCID: PMC4683085.
Article33. Douglas-Escobar M, Weiss MD. 2015; Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 169:397–403. DOI: 10.1001/jamapediatrics.2014.3269. PMID: 25685948.34. Shen R, Jia R, Liu W, Lin Q, Hai Y, He Z. 2015; The function and regulation of BMP6 in various kinds of stem cells. Curr Pharm Des. 21:3634–3643. DOI: 10.2174/1381612821666150710150218. PMID: 26166606.
Article35. Seo J, Lee EW, Shin J, Seong D, Nam YW, Jeong M, Lee SH, Lee C, Song J. 2018; K6 linked polyubiquitylation of FADD by CHIP prevents death inducing signaling complex formation suppressing cell death. Oncogene. 37:4994–5006. DOI: 10.1038/s41388-018-0323-z. PMID: 29795330.
Article36. Song S, Tan J, Miao Y, Li M, Zhang Q. 2017; Crosstalk of autophagy and apoptosis: Involvement of the dual role of autophagy under ER stress. J Cell Physiol. 232:2977–2984. DOI: 10.1002/jcp.25785. PMID: 28067409.
Article37. Roux PP, Blenis J. 2004; ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 68:320–344. DOI: 10.1128/MMBR.68.2.320-344.2004. PMID: 15187187. PMCID: PMC419926.
Article38. Yue J, López JM. 2020; Understanding MAPK signaling pathways in apoptosis. Int J Mol Sci. 21:2346. DOI: 10.3390/ijms21072346. PMID: 32231094. PMCID: PMC7177758. PMID: ea6f3fdf31194ddd9c6b405921186f76.
Article39. Rezatabar S, Karimian A, Rameshknia V, Parsian H, Majidinia M, Kopi TA, Bishayee A, Sadeghinia A, Yousefi M, Monirialamdari M, Yousefi B. 2019; RAS/MAPK signaling functions in oxidative stress, DNA damage response and cancer progression. J Cell Physiol. 234:14951–14965. DOI: 10.1002/jcp.28334. PMID: 30811039.
Article40. Tovar-y-Romo LB, Penagos-Puig A, Ramírez-Jarquín JO. 2016; Endogenous recovery after brain damage: molecular mechanisms that balance neuronal life/death fate. J Neurochem. 136:13–27. DOI: 10.1111/jnc.13362. PMID: 26376102.
Article41. Fan C, Long Y, Wang L, Liu X, Liu Z, Lan T, Li Y, Yu SY. 2020; N-acetylcysteine rescues hippocampal oxidative stress-induced neuronal injury via suppression of p38/JNK signaling in depressed rats. Front Cell Neurosci. 14:554613. DOI: 10.3389/fncel.2020.554613. PMID: 33262689. PMCID: PMC7686549. PMID: f009af972c314dd0a083facb6ffa91d0.
Article42. Park JH, Ko J, Park YS, Park J, Hwang J, Koh HC. 2017; Clearance of damaged mitochondria through PINK1 stabilization by JNK and ERK MAPK signaling in chlorpyrifos-treated neuroblastoma cells. Mol Neurobiol. 54:1844–1857. DOI: 10.1007/s12035-016-9753-1. PMID: 26892626.
Article43. Pi R, Li W, Lee NT, Chan HH, Pu Y, Chan LN, Sucher NJ, Chang DC, Li M, Han Y. 2004; Minocycline prevents glutamate-induced apoptosis of cerebellar granule neurons by differential regulation of p38 and Akt pathways. J Neurochem. 91:1219–1230. DOI: 10.1111/j.1471-4159.2004.02796.x. PMID: 15569265.
Article44. Gorina R, Font-Nieves M, Márquez-Kisinousky L, Santalucia T, Planas AM. 2011; Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. Glia. 59:242–255. DOI: 10.1002/glia.21094. PMID: 21125645.
Article45. Cuadrado A, Nebreda AR. 2010; Mechanisms and functions of p38 MAPK signalling. Biochem J. 429:403–417. DOI: 10.1042/BJ20100323. PMID: 20626350.
Article46. Katagiri K, Matsuzawa A, Ichijo H. 2010; Regulation of apoptosis signal-regulating kinase 1 in redox signaling. Methods Enzymol. 474:277–288. DOI: 10.1016/S0076-6879(10)74016-7. PMID: 20609916.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nectandrin A Enhances the BMP-Induced Osteoblastic Differentiation and Mineralization by Activation of p38 MAPK-Smad Signaling Pathway
- Quercetin induces cell death by caspase-dependent and p38 MAPK pathway in EGFR mutant lung cancer cells
- Podophyllotoxin Induces ROS-Mediated Apoptosis and Cell Cycle Arrest in Human Colorectal Cancer Cells via p38 MAPK Signaling
- Propofol attenuates hydrogenperoxide-induced apoptosis in human umbilical vein endothelial cells via multiple signaling pathways
- Anthraquinone Glycoside Aloin Induces Osteogenic Initiation of MC3T3-E1 Cells: Involvement of MAPK Mediated Wnt and Bmp Signaling