Kosin Med J.
2016 Jun;31(1):30-40. 10.7180/kmj.2016.31.1.30.
Quercetin induces cell death by caspase-dependent and p38 MAPK pathway in EGFR mutant lung cancer cells
- Affiliations
-
- 1Department of Molecular Biology and Immunology, College of Medicine, Kosin University, Busan, Korea. kimyh@kosin.ac.kr
- KMID: 2328716
- DOI: http://doi.org/10.7180/kmj.2016.31.1.30
Abstract
OBJECTIVES
The aim of this study was whether quercetin induces cell death by caspase and MAPK signaling pathway in EGFR mutant lung cancer cells.
METHODS
PC-9 cells, EGFR mutant lung cancer cells, were treated various times and concentrations of quercetin and harvested and measured using MTT assay, DNA fragmentation, Western blotting, and FACS analysis.
RESULTS
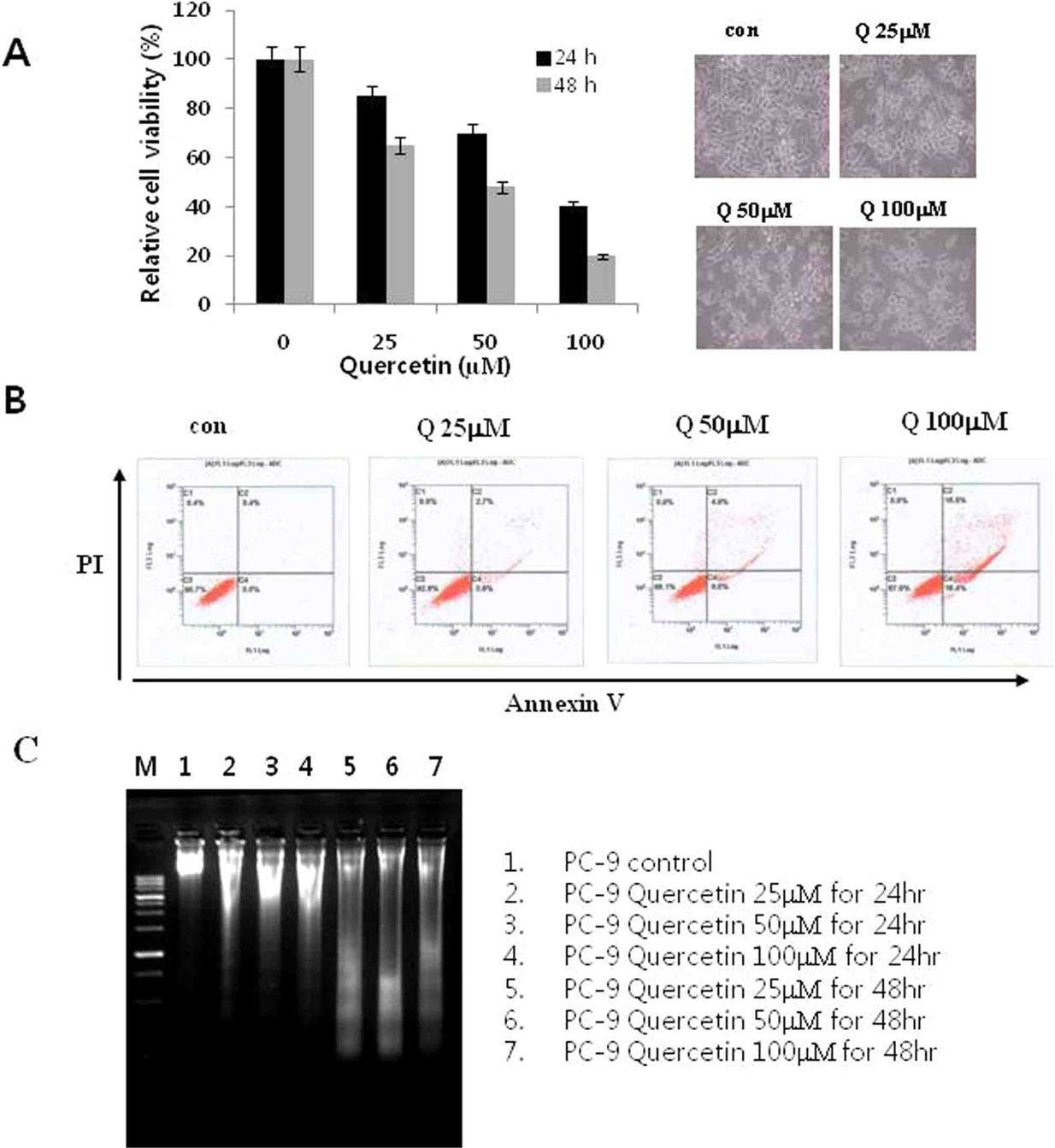
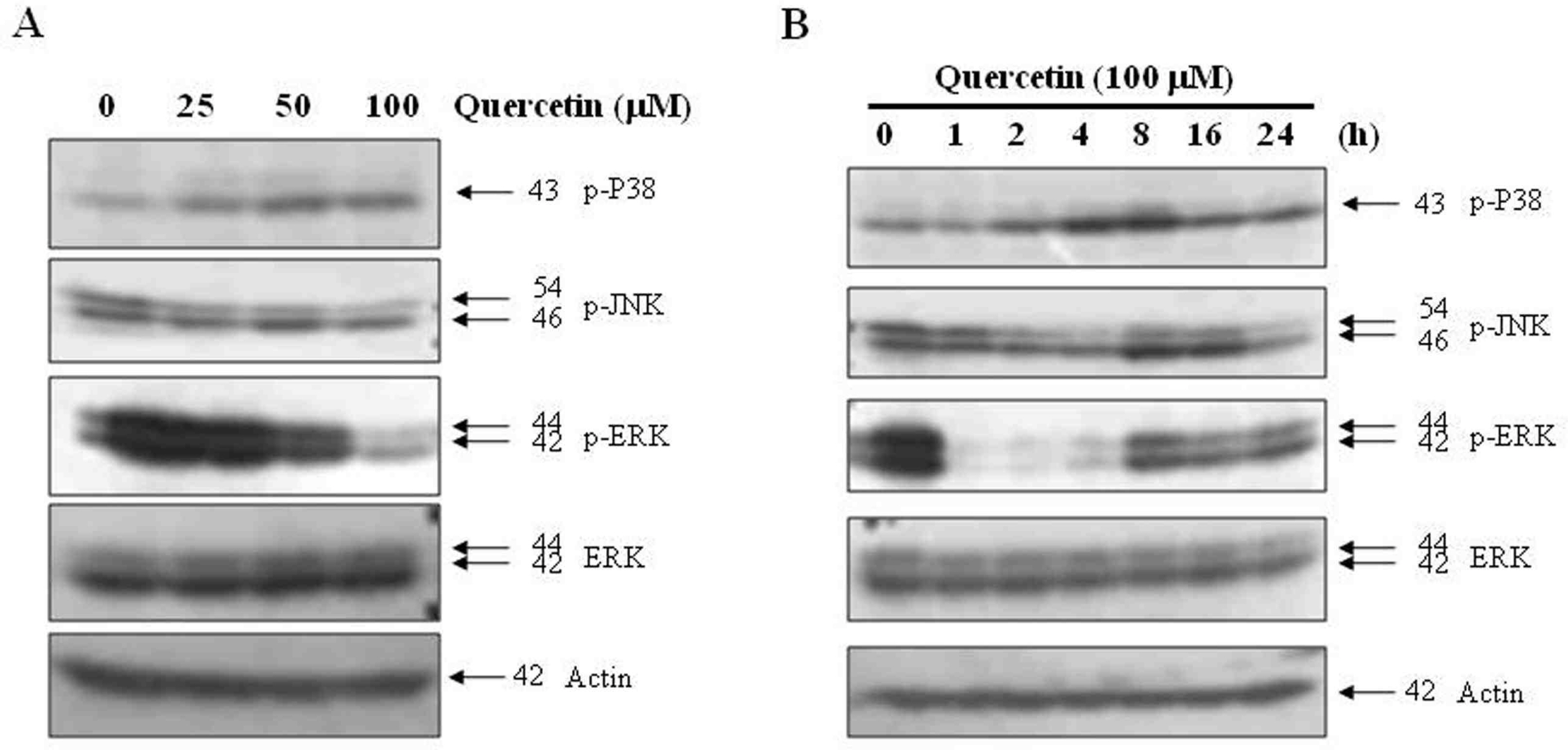
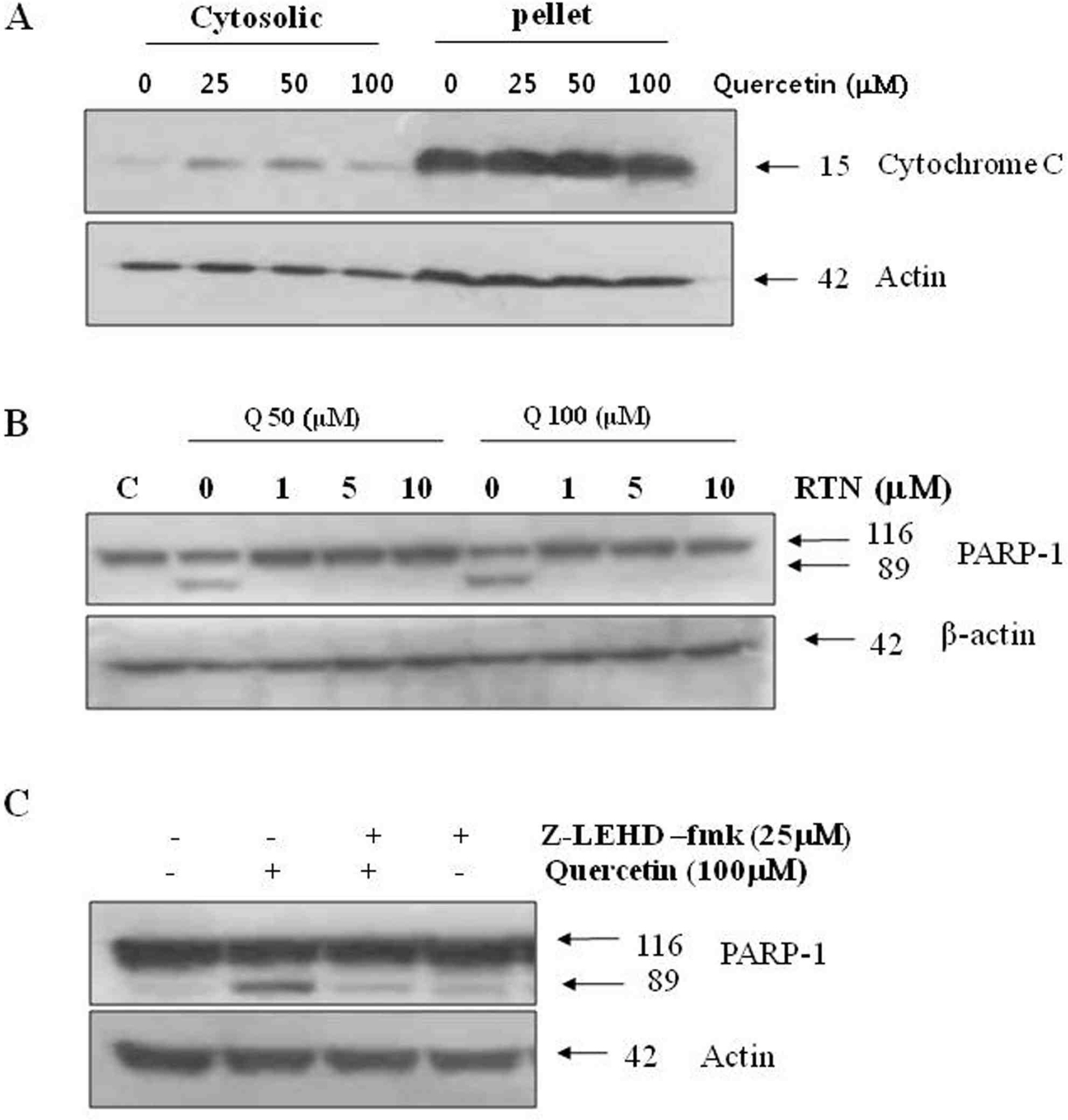
Treatment with quercetin in PC-9 cells resulted in inhibition of cell growth through apoptosis. Quercetin-induced apoptosis was associated with caspase-dependent manner. Quercetin also significantly increased levels of phosphor-p38 and decreased levels of phosphor-ERK, indicating that quercetin induces p38 MAPK signaling pathway in PC-9 cells. Quecetin treatment also generated the release of cytochrome c in PC-9 cells; however, pretreatment with rotenone or z-LEHD-fmk, significantly attenuated quercetin-induced apoptosis.
CONCLUSIONS
Our data indicate that quercetin exhibits EGFR mutant lung cancer effects through apoptosis by caspase dependent and mitochondrial pathway.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Socinski MA. Addressing the optimal duration of therapy in advanced, metastatic non-small-cell lung cancer. In: Perry MC, eds. American Society of Clinical Oncology Education Book. Alexanderia: Lisa Greaves;2003. p. 144–52.2. Nocholosn RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001; 37:S9–15.3. Wä tjen W, Michels G, Steffan B, Niering P, Chovolou Y, Kampkö tter A, et al. Low concentrations of flavonoids are protective in rat H4IIE cells whereas high concentrations cause DNA damage and apoptosis. J Nutr. 2005; 135:525–31.4. Vargas AJ, Burd R. Hormesis and synergy: pathways and mechanisms of quercetin in cancer prevention and management. Nutr Rev. 2010; 68:418–28.
Article5. Chirumbolo S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm. Allergy Drug Targets. 2010; 9:263–85.6. Ishizawa K, Yoshizumi M, Kawai Y, Terao J, Kihira Y, Ikeda Y, et al. Pharmacology in health food: metabolism of quercetin in vivo and its protective effect against arteriosclerosis. J Pharmacol Sci. 2011; 115:466–70.
Article7. Perez-Vizcaino F, Duarte J, Jimenez R, Santos-Buelga C, Osuna A. Antihypertensive effects of the flavonoid quercetin. Pharmacol Rep. 2009; 61:67–75.
Article8. Ossola B, Kää riä inen TM, Mä nnistö PT. The multiple faces of quercetin in neuroprotection. Expert Opin Drug Saf. 2009; 8:397–409.
Article9. Kelsey NA, Wilkins HM, Linseman DA. Nutraceutical antioxidants as novel neuroprotective agents. Molecules. 2010; 15:7792–814.
Article10. Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008; 269:315–25.
Article11. Seufi AM, Ibrahim SS, Elmaghraby TK, Hafez EE. Preventive effect of the flavonoid, quercetin, on hepatic cancer in rats via oxidant/antioxidant activity: molecular and histological evidences. J Exp Clin Cancer Res. 2009; 28:80–7.
Article12. Priyadarsini RV, Vinothini G, Murugan RS, Manikandan P, Nagini S. The flavonoid quercetin modulates the hallmark capabilities of hamster buccal pouch tumors. Nutr Cancer. 2011; 63:218–26.
Article13. Camargo CA, da Silva ME, da Silva RA, Justo GZ, Gomes-Marcondes MC, Aoyama H. Inhibition of tumor growth by quercetin with increase of survival and prevention of cachexia in Walker 256 tu-mor-bearing rats. Biochem Biophys Res Commun. 2011; 406:638–42.
Article14. Mendoza EE, Burd R. Quercetin as a systemic che-mopreventative agent: structural and functional mechanisms. Mini Rev Med Chem. 2011; 11:1216–21.15. Djuric Z, Severson RK, Kato I. Association of dietary quercetin with reduced risk of proximal colon cancer. Nutr Cancer. 2012; 64:351–60.
Article16. Cincin ZB, Unlu M, Kiran B, Bireller ES, Baran Y, Cakmakoglu B. Molecular mechanisms of quer-citrin-induced apoptosis in non-small cell lung cancer. Arch Med Res. 2014; 45:445–54.
Article17. Kim YH, Lee DH, Jeong JH, Guo ZS, Lee YJ. Quercetin augments TRAIL-induced apoptotic death: involvement of the ERK signal transduction pathway. Biochem Pharmacol. 2008; 75:1946–58.
Article18. Kim YH, Lee YJ. TRAIL apoptosis is enhanced by quercetin through Akt dephosphorylation. J Cell Biochem. 2007; 100:998–1009.
Article19. Elimore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007; 35:495–516.
Article20. Wang J, Yu J. Cancer cell killing via ROS: to increase or decrease, that is the question. Cancer Biol Ther. 2008; 7:1875–84.
Article21. Galluzzo P, Martini C, Bulzomi P, Leone S, Bolli A, Pallottini V, et al. Quercetin-induced apoptotic cascade in cancer cells: antioxidant versus estrogen receptor alpha-dependent mechanisms. Mol Nutr Food Res 2009;53:699–708.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cell Death Induction Mechanism of Non-small Cell Lung Cancer Cell Line, NCI-H1703 by Docetaxel
- Melatonin Induces Apoptotic Cell Death via p53 in LNCaP Cells
- Role of p53 and p38 MAPK on Doxorubicin and Lovastatin-induced Apoptosis in Colon Cancer Cells
- BMP‑6 Attenuates Oxygen and Glucose Deprivation-Induced Apoptosis in Human Neural Stem Cells through Inhibiting p38 MAPK Signaling Pathway
- Arctigenin induces caspase-dependent apoptosis in FaDu human pharyngeal carcinoma cells