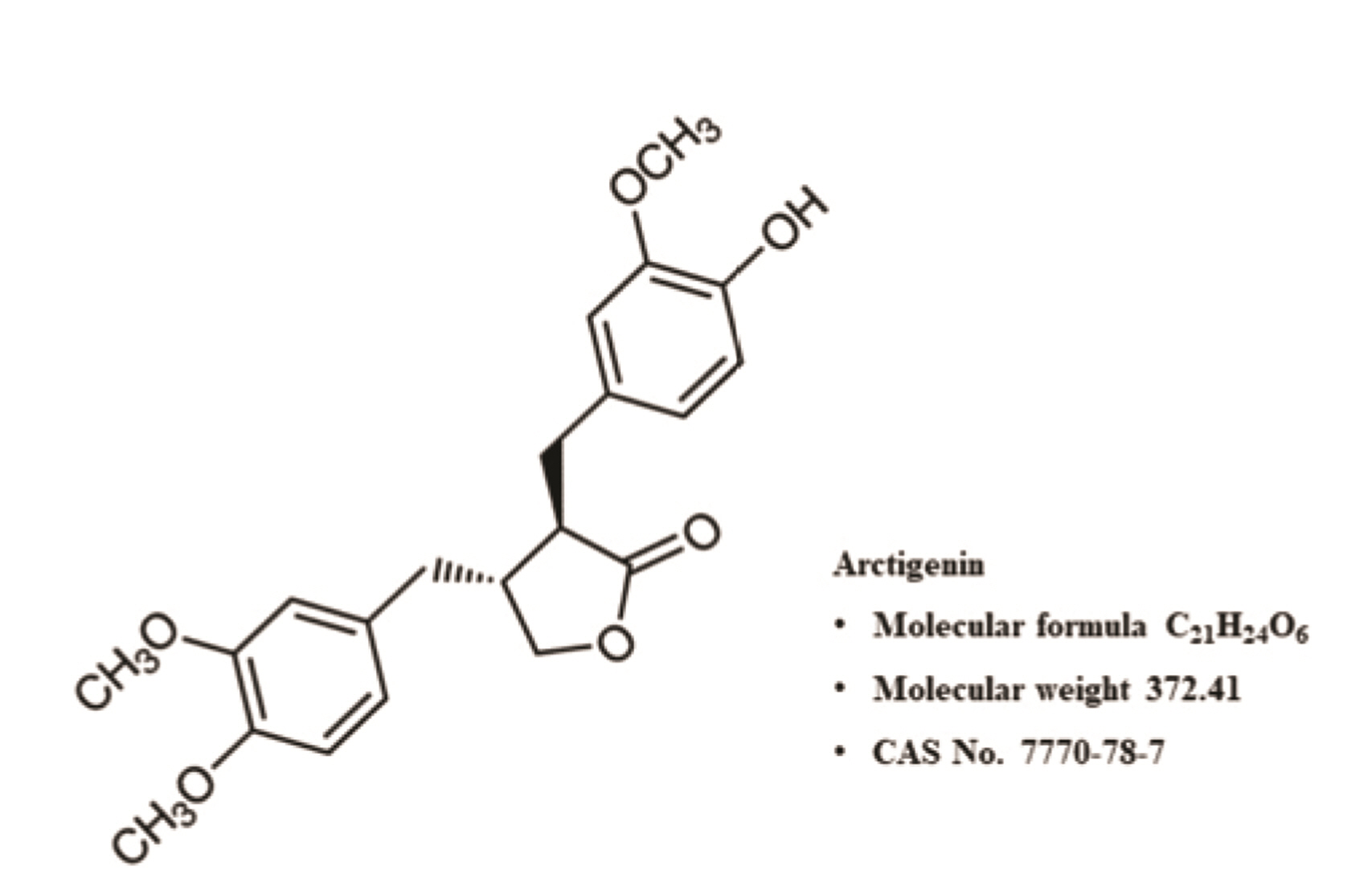
Korean J Physiol Pharmacol.
2022 Nov;26(6):447-456. 10.4196/kjpp.2022.26.6.447.
Arctigenin induces caspase-dependent apoptosis in FaDu human pharyngeal carcinoma cells
- Affiliations
-
- 1The Institute of Dental Science, Chosun University, Gwangju 61452, Korea
- 2Department of Integrative Biological Sciences & BK21 FOUR Educational Research Group for Ageassociated Disorder Control Technology, Chosun University, Gwangju 61452, Korea
- KMID: 2534521
- DOI: http://doi.org/10.4196/kjpp.2022.26.6.447
Abstract
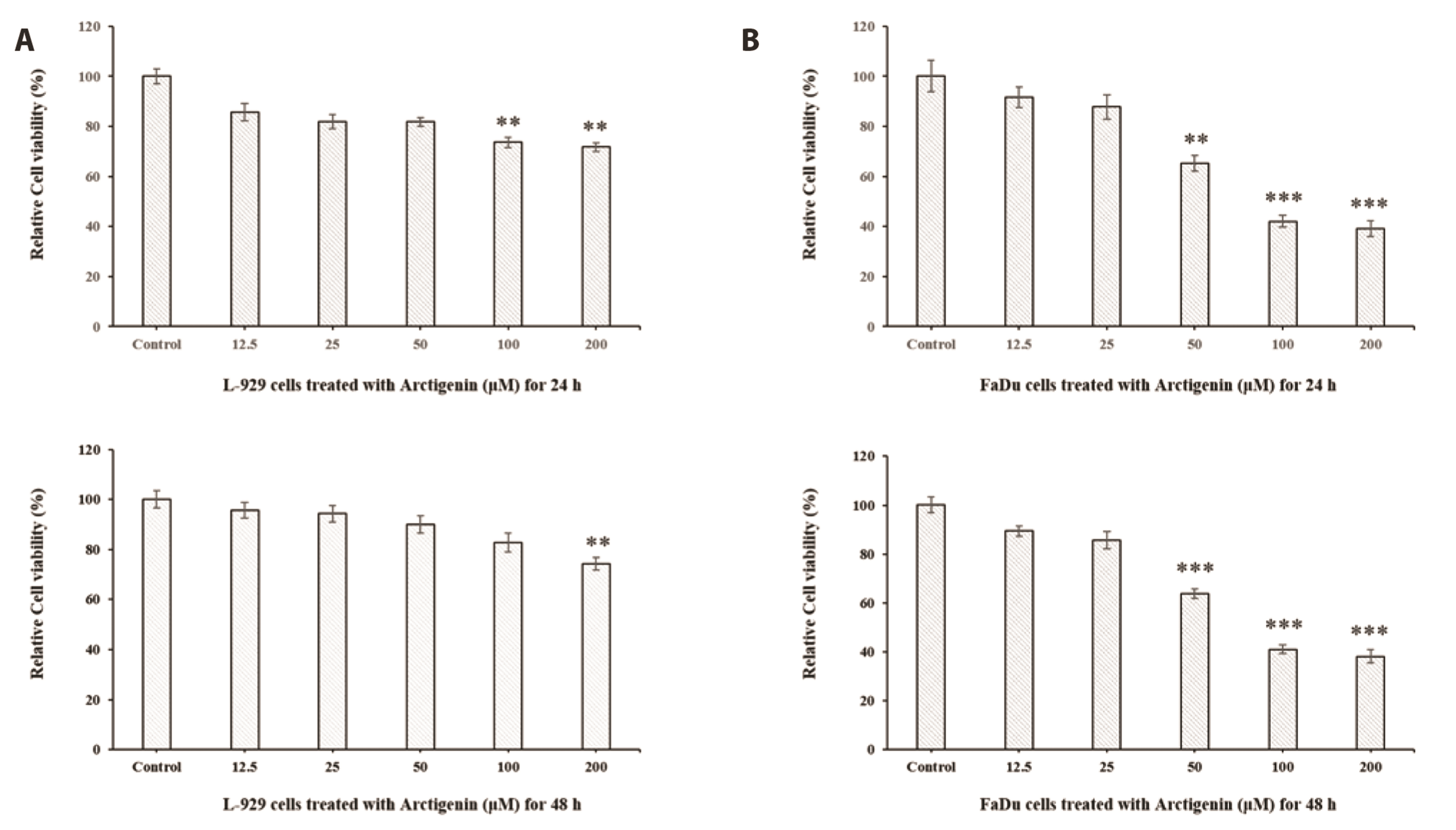
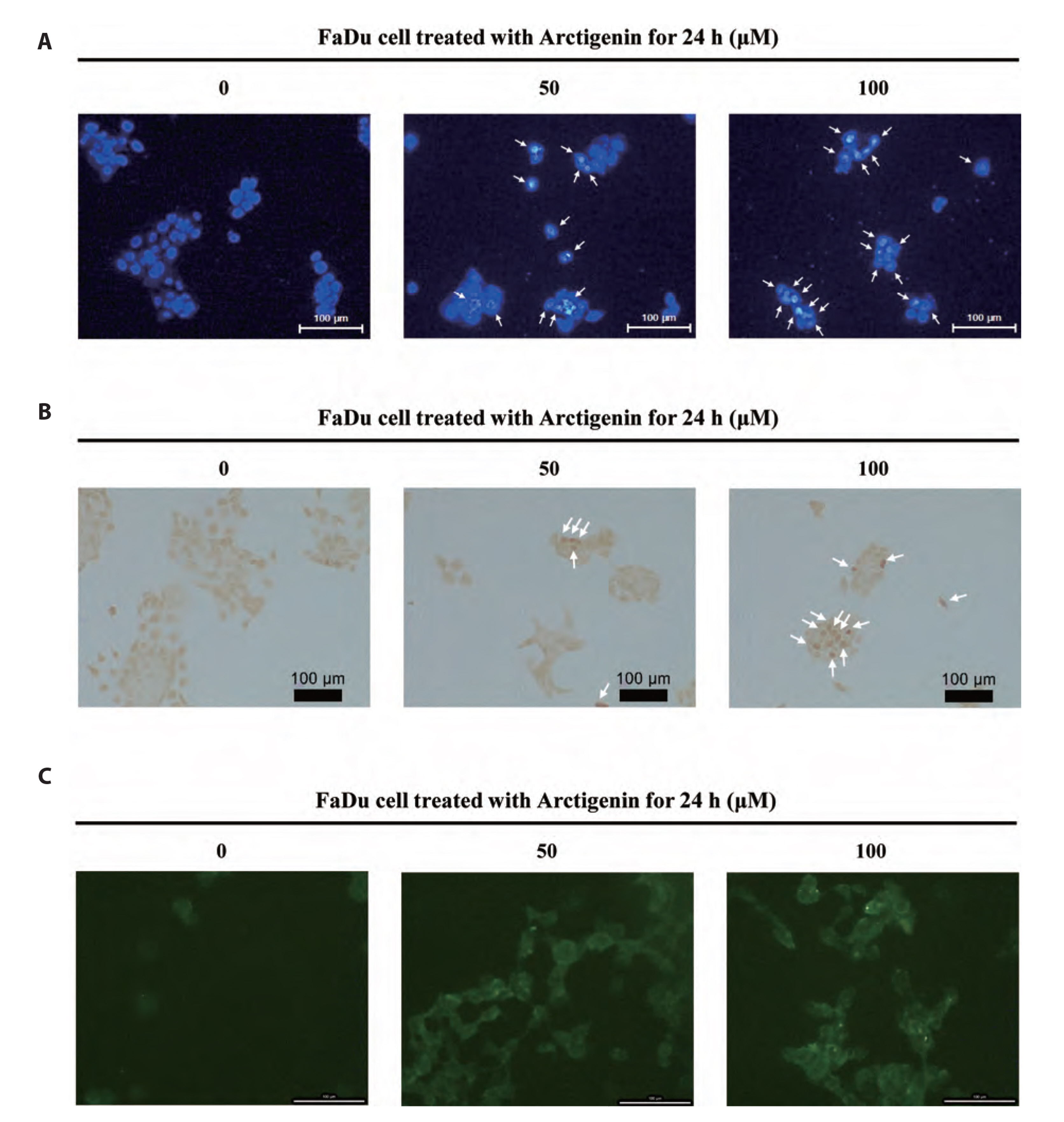
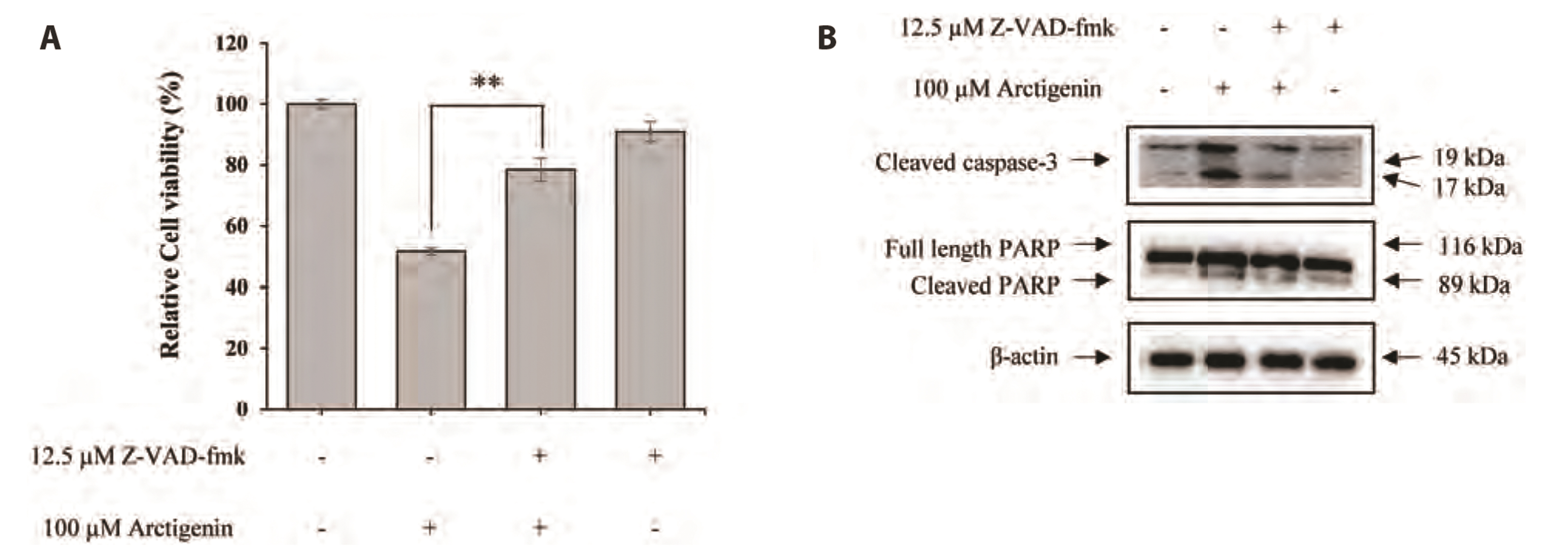
- The present study was carried out to investigate the effect of Arctigenin on cell growth and the mechanism of cell death elicited by Arctigenin were examined in FaDu human pharyngeal carcinoma cells. To determine the apoptotic activity of Arctigenin in FaDu human pharyngeal carcinoma cells, cell viability assay, DAPI staining, caspase activation analysis, and immunoblotting were performed. Arctigenin inhibited the growth of cells in a dose-dependent manner and induced nuclear condensation and fragmentation. Arctigenin-treated cells showed caspase-3/7 activation and increased apoptosis versus control cells. FasL, a death ligand associated with extrinsic apoptotic signaling pathways, was up-regulated by Arctigenin treatment. Moreover, caspase-8, a part of the extrinsic apoptotic pathway, was activated by Arctigenin treatments. Expressions of anti-apoptotic factors such as Bcl-2 and Bcl-xL, components of the mitochondria-dependent intrinsic apoptosis pathway, significantly decreased following Arctigenin treatment. The expressions of pro-apoptotic factors such as BAX, BAD and caspase-9, and tumor suppressor -53 increased by Arctigenin treatments. In addition, Arctigenin activated caspase-3 and poly (ADP-ribose) polymerase (PARP) induced cell death. Arctigenin also inhibited the proliferation of FaDu cells by the suppression of p38, NF-κB, and Akt signaling pathways. These results suggest that Arctigenin may inhibit cell proliferation and induce apoptotic cell death in FaDu human pharyngeal carcinoma cells through both the mitochondria-mediated intrinsic pathway and the death receptormediated extrinsic pathway.
Figure
Reference
-
1. Warnakulasuriya S. 2009; Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 45:309–316. DOI: 10.1016/j.oraloncology.2008.06.002. PMID: 18804401. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=67349186933&origin=inward.
Article2. Kalavrezos N, Scully C. 2015; Mouth cancer for clinicians. Part 2: epidemiology. Dent Update. 42:354–356. 358–359. DOI: 10.12968/denu.2015.42.4.354. PMID: 26062260.
Article3. Sun F, Li D, Wang C, Peng C, Zheng H, Wang X. 2019; Acacetin-induced cell apoptosis in head and neck squamous cell carcinoma cells: evidence for the role of muscarinic M3 receptor. Phytother Res. 33:1551–1561. DOI: 10.1002/ptr.6343. PMID: 31066474. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85065512110&origin=inward.
Article4. Rettig EM, D'Souza G. 2015; Epidemiology of head and neck cancer. Surg Oncol Clin N Am. 24:379–396. DOI: 10.1016/j.soc.2015.03.001. PMID: 25979389. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84929504129&origin=inward.
Article5. Pezzuto F, Buonaguro L, Caponigro F, Ionna F, Starita N, Annunziata C, Buonaguro FM, Tornesello ML. 2015; Update on head and neck cancer: current knowledge on epidemiology, risk factors, molecular features and novel therapies. Oncology. 89:125–136. DOI: 10.1159/000381717. PMID: 25967534. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84942190711&origin=inward.
Article6. Kundu SK, Nestor M. 2012; Targeted therapy in head and neck cancer. Tumour Biol. 33:707–721. DOI: 10.1007/s13277-012-0350-2. PMID: 22373581. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84863587948&origin=inward.
Article7. Sacks PG. 1996; Cell, tissue and organ culture as in vitro models to study the biology of squamous cell carcinomas of the head and neck. Cancer Metastasis Rev. 15:27–51. DOI: 10.1007/BF00049486. PMID: 8842478. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0029929233&origin=inward.
Article8. Todd R, Donoff RB, Wong DT. 1997; The molecular biology of oral carcinogenesis: toward a tumor progression model. J Oral Maxillofac Surg. 55:613–623. discussion 623–625. DOI: 10.1016/S0278-2391(97)90495-X. PMID: 9191644. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0030926441&origin=inward.
Article9. Links M, Lewis C. 1999; Chemoprotectants: a review of their clinical pharmacology and therapeutic efficacy. Drugs. 57:293–308. DOI: 10.2165/00003495-199957030-00003. PMID: 10193684. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033014520&origin=inward.10. Goldar S, Khaniani MS, Derakhshan SM, Baradaran B. 2015; Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J Cancer Prev. 16:2129–2144. DOI: 10.7314/APJCP.2015.16.6.2129. PMID: 25824729. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84929224850&origin=inward.
Article11. Rahman MA, Hannan MA, Dash R, Rahman MH, Islam R, Uddin MJ, Sohag AAM, Rahman MH, Rhim H. 2021; Phytochemicals as a complement to cancer chemotherapy: pharmacological modulation of the autophagy-apoptosis pathway. Front Pharmacol. 12:639628. DOI: 10.3389/fphar.2021.639628. PMID: 34025409. PMCID: PMC8138161. PMID: 8e76401c7d1f4cf1b752c609c09dc5db. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85106167533&origin=inward.
Article12. Sak K. 2012; Chemotherapy and dietary phytochemical agents. Chemother Res Pract. 2012:282570. DOI: 10.1155/2012/282570. PMID: 23320169. PMCID: PMC3539428.
Article13. Alfarouk KO, Stock CM, Taylor S, Walsh M, Muddathir AK, Verduzco D, Bashir AH, Mohammed OY, Elhassan GO, Harguindey S, Reshkin SJ, Ibrahim ME, Rauch C. 2015; Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 15:71. DOI: 10.1186/s12935-015-0221-1. PMID: 26180516. PMCID: PMC4502609. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84937028711&origin=inward.
Article14. Tompkins KD, Thorburn A. 2019; Regulation of apoptosis by autophagy to enhance cancer therapy. Yale J Biol Med. 92:707–718. PMID: 31866785. PMCID: PMC6913805. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85077163064&origin=inward.15. Wang X, Zhang H, Chen X. 2019; Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2:141–160. DOI: 10.20517/cdr.2019.10. PMID: 34322663. PMCID: PMC8315569. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85103071967&origin=inward.
Article16. Kaufmann SH, Earnshaw WC. 2000; Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 256:42–49. DOI: 10.1006/excr.2000.4838. PMID: 10739650. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0034630167&origin=inward.
Article17. Reed JC. 2001; Apoptosis-regulating proteins as targets for drug discovery. Trends Mol Med. 7:314–319. DOI: 10.1016/S1471-4914(01)02026-3. PMID: 11425640. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0034953632&origin=inward.
Article18. Punia R, Raina K, Agarwal R, Singh RP. 2017; Acacetin enhances the therapeutic efficacy of doxorubicin in non-small-cell lung carcinoma cells. PLoS One. 12:e0182870. DOI: 10.1371/journal.pone.0182870. PMID: 28859099. PMCID: PMC5578506. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85029188611&origin=inward.
Article19. Gao Q, Yang M, Zuo Z. 2018; Overview of the anti-inflammatory effects, pharmacokinetic properties and clinical efficacies of arctigenin and arctiin from Arctium lappa L. Acta Pharmacol Sin. 39:787–801. DOI: 10.1038/aps.2018.32. PMID: 29698388. PMCID: PMC5943914. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85046671767&origin=inward.
Article20. Yang Z, Liu N, Huang B, Wang Y, Hu Y, Zhu Y. 2005; Effect of anti-influenza virus of Arctigenin in vivo. Zhong Yao Cai. 28:1012–1014. Chinese. PMID: 16514891. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33748874508&origin=inward.21. Tsai WJ, Chang CT, Wang GJ, Lee TH, Chang SF, Lu SC, Kuo YC. 2011; Arctigenin from Arctium lappa inhibits interleukin-2 and interferon gene expression in primary human T lymphocytes. Chin Med. 6:12. DOI: 10.1186/1749-8546-6-12. PMID: 21435270. PMCID: PMC3076299. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79953092808&origin=inward.
Article22. Yao X, Li G, Lü C, Xu H, Yin Z. 2012; Arctigenin promotes degradation of inducible nitric oxide synthase through CHIP-associated proteasome pathway and suppresses its enzyme activity. Int Immunopharmacol. 14:138–144. DOI: 10.1016/j.intimp.2012.06.017. PMID: 22770942. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84864075049&origin=inward.
Article23. Lee JY, Kim CJ. 2010; Arctigenin, a phenylpropanoid dibenzylbutyrolactone lignan, inhibits type I-IV allergic inflammation and pro-inflammatory enzymes. Arch Pharm Res. 33:947–957. DOI: 10.1007/s12272-010-0619-1. PMID: 20607501. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77954485451&origin=inward.
Article24. Kang HS, Lee JY, Kim CJ. 2008; Anti-inflammatory activity of arctigenin from Forsythiae Fructus. J Ethnopharmacol. 116:305–312. DOI: 10.1016/j.jep.2007.11.030. PMID: 18180122. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=39149122828&origin=inward.
Article25. Lu Z, Cao S, Zhou H, Hua L, Zhang S, Cao J. 2015; Mechanism of arctigenin-induced specific cytotoxicity against human hepatocellular carcinoma cell lines: Hep G2 and SMMC7721. PLoS One. 10:e0125727. DOI: 10.1371/journal.pone.0125727. PMID: 25933104. PMCID: PMC4416797. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84928776796&origin=inward.
Article26. He Y, Fan Q, Cai T, Huang W, Xie X, Wen Y, Shi Z. 2018; Molecular mechanisms of the action of arctigenin in cancer. Biomed Pharmacother. 108:403–407. DOI: 10.1016/j.biopha.2018.08.158. PMID: 30236849. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85053399921&origin=inward.
Article27. Wang HQ, Jin JJ, Wang J. 2014; Arctigenin enhances chemosensitivity to cisplatin in human nonsmall lung cancer H460 cells through downregulation of survivin expression. J Biochem Mol Toxicol. 28:39–45. DOI: 10.1002/jbt.21533. PMID: 24395429. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84891632136&origin=inward.
Article28. Maxwell T, Chun SY, Lee KS, Kim S, Nam KS. 2017; The anti-metastatic effects of the phytoestrogen arctigenin on human breast cancer cell lines regardless of the status of ER expression. Int J Oncol. 50:727–735. DOI: 10.3892/ijo.2016.3825. PMID: 28035371. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85013040741&origin=inward.
Article29. Kim JS, Cho IA, Kang KR, Lim H, Kim TH, Yu SK, Kim HJ, Lee SA, Moon SM, Chun HS, Kim CS, Kim DK. 2019; Reversine induces caspase-dependent apoptosis of human osteosarcoma cells through extrinsic and intrinsic apoptotic signaling pathways. Genes Genomics. 41:657–665. DOI: 10.1007/s13258-019-00790-1. PMID: 30953339. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85064266609&origin=inward.
Article30. Park MG, Kim JS, Park SY, Lee SA, Kim HJ, Kim CS, Kim JS, Chun HS, Park JC, Kim DK. 2014; MicroRNA-27 promotes the differentiation of odontoblastic cell by targeting APC and activating Wnt/β-catenin signaling. Gene. 538:266–272. DOI: 10.1016/j.gene.2014.01.045. PMID: 24487055. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84893935584&origin=inward.
Article31. Hadden JW. 1997; The immunopharmacology of head and neck cancer: an update. Int J Immunopharmacol. 19:629–644. DOI: 10.1016/S0192-0561(97)00063-5. PMID: 9669203. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0344447055&origin=inward.
Article32. Hensley P, Mishra M, Kyprianou N. 2013; Targeting caspases in cancer therapeutics. Biol Chem. 394:831–843. DOI: 10.1515/hsz-2013-0128. PMID: 23509217. PMCID: PMC3721733. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84881177777&origin=inward.
Article33. Ray RS, Agrawal N, Sharma A, Hans RK. 2008; Use of L-929 cell line for phototoxicity assessment. Toxicol In Vitro. 22:1775–1781. DOI: 10.1016/j.tiv.2008.06.015. PMID: 18657604. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=51749101270&origin=inward.
Article34. Jiang Y, Liu J, Hong W, Fei X, Liu R. 2020; Arctigenin inhibits glioblastoma proliferation through the AKT/mTOR pathway and induces autophagy. Biomed Res Int. 2020:3542613. DOI: 10.1155/2020/3542613. PMID: 33015162. PMCID: PMC7512051. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85092488454&origin=inward.
Article35. Yang S, Ma J, Xiao J, Lv X, Li X, Yang H, Liu Y, Feng S, Zhang Y. 2012; Arctigenin anti-tumor activity in bladder cancer T24 cell line through induction of cell-cycle arrest and apoptosis. Anat Rec (Hoboken). 295:1260–1266. DOI: 10.1002/ar.22497. PMID: 22619087. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84863549883&origin=inward.36. Toné S, Sugimoto K, Tanda K, Suda T, Uehira K, Kanouchi H, Samejima K, Minatogawa Y, Earnshaw WC. 2007; Three distinct stages of apoptotic nuclear condensation revealed by time-lapse imaging, biochemical and electron microscopy analysis of cell-free apoptosis. Exp Cell Res. 313:3635–3644. DOI: 10.1016/j.yexcr.2007.06.018. PMID: 17643424. PMCID: PMC2705844. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34548696434&origin=inward.
Article37. Creagh EM, Conroy H, Martin SJ. 2003; Caspase-activation pathways in apoptosis and immunity. Immunol Rev. 193:10–21. DOI: 10.1034/j.1600-065X.2003.00048.x. PMID: 12752666. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0037833742&origin=inward.
Article38. Herrnring C, Reimer T, Jeschke U, Makovitzky J, Krüger K, Gerber B, Kabelitz D, Friese K. 2000; Expression of the apoptosis-inducing ligands FasL and TRAIL in malignant and benign human breast tumors. Histochem Cell Biol. 113:189–194. DOI: 10.1007/s004180050438. PMID: 10817673. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0034036052&origin=inward.
Article39. Kluck RM, Martin SJ, Hoffman BM, Zhou JS, Green DR, Newmeyer DD. 1997; Cytochrome c activation of CPP32-like proteolysis plays a critical role in a Xenopus cell-free apoptosis system. EMBO J. 16:4639–4649. DOI: 10.1093/emboj/16.15.4639. PMID: 9303308. PMCID: PMC1170090. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0030741528&origin=inward.
Article40. Kroemer G, Reed JC. 2000; Mitochondrial control of cell death. Nat Med. 6:513–519. DOI: 10.1038/74994. PMID: 10802706. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0034068601&origin=inward.
Article41. Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. 2001; Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 292:727–730. DOI: 10.1126/science.1059108. PMID: 11326099. PMCID: PMC3049805. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035957653&origin=inward.
Article42. Han YH, Kee JY, Kim DS, Mun JG, Jeong MY, Park SH, Choi BM, Park SJ, Kim HJ, Um JY, Hong SH. 2016; Arctigenin inhibits lung metastasis of colorectal cancer by regulating cell viability and metastatic phenotypes. Molecules. 21:1135. DOI: 10.3390/molecules21091135. PMID: 27618887. PMCID: PMC6272973. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84987858588&origin=inward.
Article43. Yue J, López JM. 2020; Understanding MAPK signaling pathways in apoptosis. Int J Mol Sci. 21:2346. DOI: 10.3390/ijms21072346. PMID: 32231094. PMCID: PMC7177758. PMID: ea6f3fdf31194ddd9c6b405921186f76. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85082791079&origin=inward.
Article44. Royuela M, Arenas MI, Bethencourt FR, Sánchez-Chapado M, Fraile B, Paniagua R. 2002; Regulation of proliferation/apoptosis equilibrium by mitogen-activated protein kinases in normal, hyperplastic, and carcinomatous human prostate. Hum Pathol. 33:299–306. DOI: 10.1053/hupa.2002.32227. PMID: 11979370. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036235788&origin=inward.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of cimifugin on cell growth inhibition and cell apoptosis induction in fadu human pharyngeal squamous cell carcinoma
- Apoptotic effects of hyperoside on FaDu human pharyngeal carcinoma cells
- Inhibition of cell growth and induction of apoptosis by bilobalide in FaDu human pharyngeal squamous cell carcinoma
- Inhibition of cell growth and induction of apoptosis by acacetin in FaDu human pharyngeal carcinoma cells
- Apoptotic effect of betulinic acid in FaDu human head and neck squamous cell carcinomas