J Pathol Transl Med.
2019 Mar;53(2):94-103. 10.4132/jptm.2019.01.14.
Guanabenz Acetate Induces Endoplasmic Reticulum Stress–Related Cell Death in Hepatocellular Carcinoma Cells
- Affiliations
-
- 1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. jangsejin@amc.seoul.kr, d890075@gmail.com
- 2Asan institute for Life Science, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Asan Liver Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2465448
- DOI: http://doi.org/10.4132/jptm.2019.01.14
Abstract
- BACKGROUND
Development of chemotherapeutics for the treatment of advanced hepatocellular carcinoma (HCC) has been lagging. Screening of candidate therapeutic agents by using patient-derived preclinical models may facilitate drug discovery for HCC patients.
METHODS
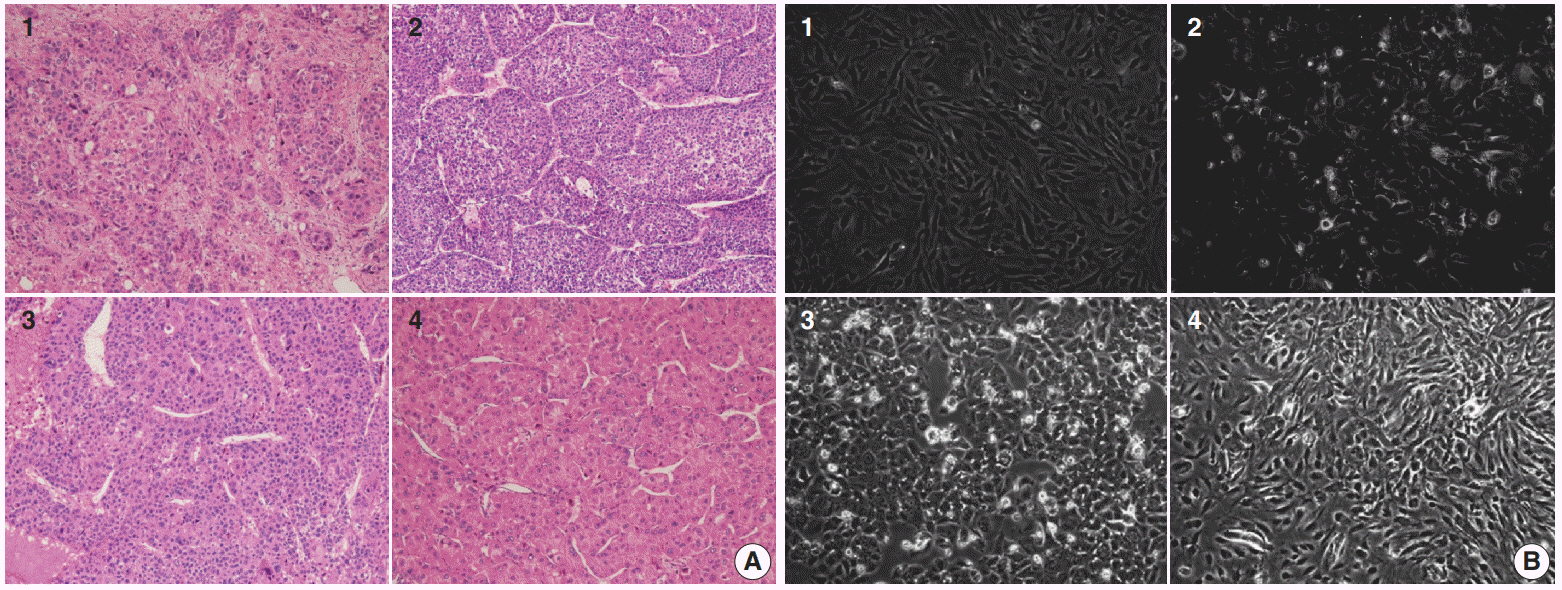
Four primary cultured HCC cells from surgically resected tumor tissues and six HCC cell lines were used for high-throughput screening of 252 drugs from the Prestwick Chemical Library. The efficacy and mechanisms of action of the candidate anti-cancer drug were analyzed via cell viability, cell cycle assays, and western blotting.
RESULTS
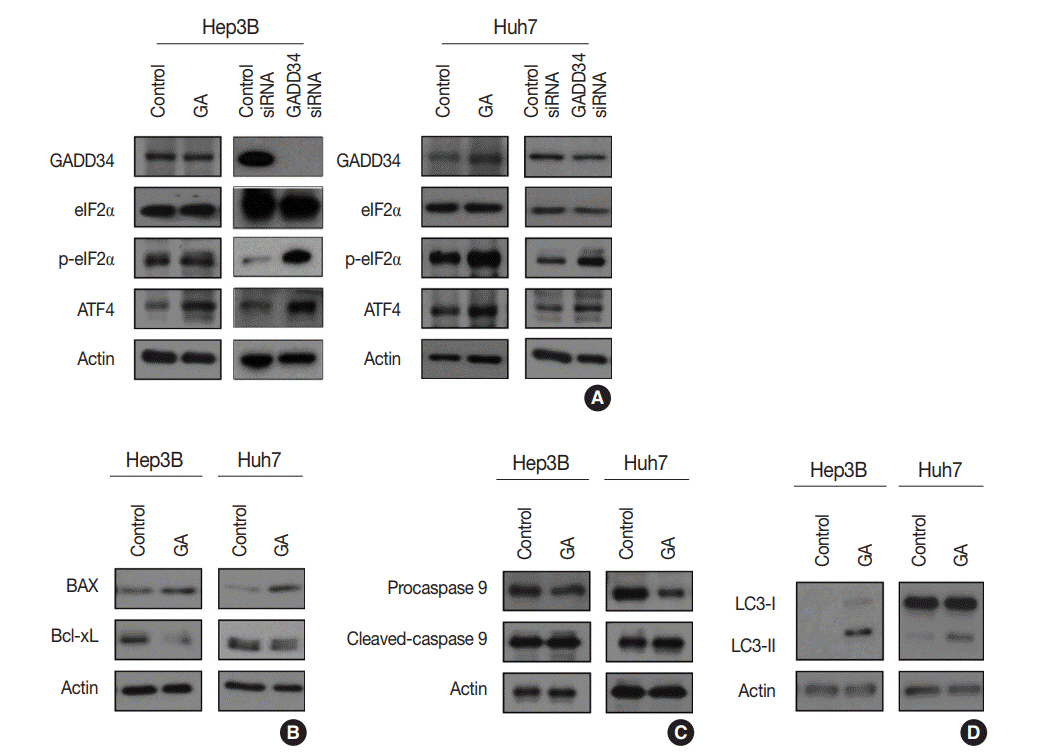
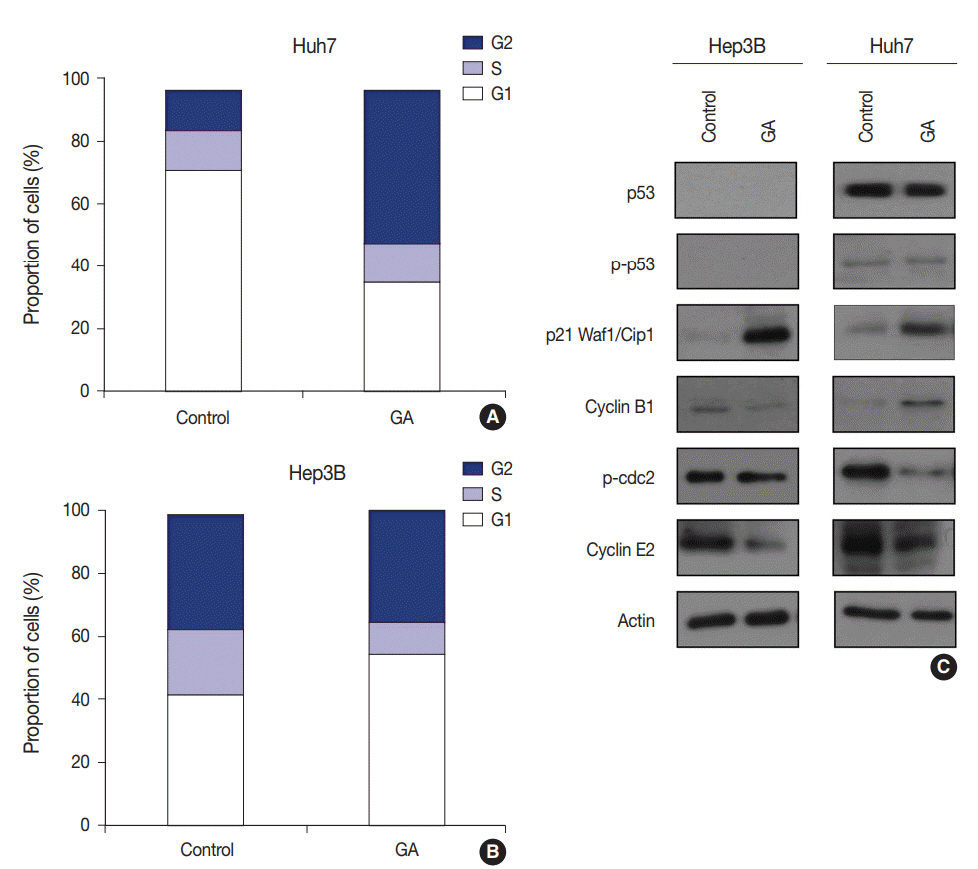
Guanabenz acetate, which has been used as an antihypertensive drug, was screened as a candidate anti-cancer agent for HCC through a drug sensitivity assay by using the primary cultured HCC cells and HCC cell lines. Guanabenz acetate reduced HCC cell viability through apoptosis and autophagy. This occurred via inhibition of growth arrest and DNA damage-inducible protein 34, increased phosphorylation of eukaryotic initiation factor 2α, increased activating transcription factor 4, and cell cycle arrest.
CONCLUSIONS
Guanabenz acetate induces endoplasmic reticulum stress-related cell death in HCC and may be repositioned as an anti-cancer therapeutic agent for HCC patients.
Keyword
MeSH Terms
-
Activating Transcription Factor 4
Apoptosis
Autophagy
Blotting, Western
Carcinoma, Hepatocellular*
Cell Cycle
Cell Cycle Checkpoints
Cell Death*
Cell Line
Cell Survival
DNA
Drug Discovery
Drug Repositioning
Endoplasmic Reticulum*
Guanabenz*
Humans
Mass Screening
Peptide Initiation Factors
Phosphorylation
Primary Cell Culture
Activating Transcription Factor 4
DNA
Guanabenz
Peptide Initiation Factors
Figure
Reference
-
1. Bosch FX, Ribes J, Cléries R, Díaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005; 9:191–211.
Article2. Shaw JJ, Shah SA. Rising incidence and demographics of hepatocellular carcinoma in the USA: what does it mean? Expert Rev Gastroenterol Hepatol. 2011; 5:365–70.
Article3. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003; 362:1907–17.
Article4. Karaman B, Battal B, Sari S, Verim S. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol. 2014; 20:18059–60.
Article5. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012; 379:1245–55.
Article6. Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010; 15 Suppl 4:5–13.
Article7. Crissien AM, Frenette C. Current management of hepatocellular carcinoma. Gastroenterol Hepatol (N Y). 2014; 10:153–61.8. Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009; 69:223–40.9. Patel A, Sun W. Molecular targeted therapy in hepatocellular carcinoma: from biology to clinical practice and future. Curr Treat Options Oncol. 2014; 15:380–94.
Article10. Berk V, Kaplan MA, Tonyali O, et al. Efficiency and side effects of sorafenib therapy for advanced hepatocellular carcinoma: a retrospective study by the anatolian society of medical oncology. Asian Pac J Cancer Prev. 2013; 14:7367–9.
Article11. De Witt Hamer PC, Van Tilborg AA, Eijk PP, et al. The genomic profile of human malignant glioma is altered early in primary cell culture and preserved in spheroids. Oncogene. 2008; 27:2091–6.
Article12. Seol HS, Suh YA, Ryu YJ, et al. A patient-derived xenograft mouse model generated from primary cultured cells recapitulates patient tumors phenotypically and genetically. J Cancer Res Clin Oncol. 2013; 139:1471–80.
Article13. Seol HS, Kang HJ, Lee SI, et al. Development and characterization of a colon PDX model that reproduces drug responsiveness and the mutation profiles of its original tumor. Cancer Lett. 2014; 345:56–64.
Article14. Mitra A, Mishra L, Li S. Technologies for deriving primary tumor cells for use in personalized cancer therapy. Trends Biotechnol. 2013; 31:347–54.
Article15. Shim JS, Liu JO. Recent advances in drug repositioning for the discovery of new anticancer drugs. Int J Biol Sci. 2014; 10:654–63.
Article16. Würth R, Thellung S, Bajetto A, Mazzanti M, Florio T, Barbieri F. Drug-repositioning opportunities for cancer therapy: novel molecular targets for known compounds. Drug Discov Today. 2016; 21:190–9.
Article17. Banno K, Iida M, Yanokura M, et al. Drug repositioning for gynecologic tumors: a new therapeutic strategy for cancer. Scientific-WorldJournal. 2015; 2015:341362.
Article18. Langedijk J, Mantel-Teeuwisse AK, Slijkerman DS, Schutjens MH. Drug repositioning and repurposing: terminology and definitions in literature. Drug Discov Today. 2015; 20:1027–34.
Article19. Saxena A, Becker D, Preeshagul I, Lee K, Katz E, Levy B. Therapeutic effects of repurposed therapies in non-small cell lung cancer: what is old is new again. Oncologist. 2015; 20:934–45.
Article20. Oprea TI, Overington JP. Computational and practical aspects of drug repositioning. Assay Drug Dev Technol. 2015; 13:299–306.
Article21. Wang L, Popko B, Tixier E, Roos RP. Guanabenz, which enhances the unfolded protein response, ameliorates mutant SOD1-induced amyotrophic lateral sclerosis. Neurobiol Dis. 2014; 71:317–24.
Article22. Rozpedek W, Markiewicz L, Diehl JA, Pytel D, Majsterek I. Unfolded protein response and PERK kinase as a new therapeutic target in the pathogenesis of Alzheimer's disease. Curr Med Chem. 2015; 22:3169–84.
Article23. Shah SZ, Zhao D, Khan SH, Yang L. Unfolded protein response pathways in neurodegenerative diseases. J Mol Neurosci. 2015; 57:529–37.
Article24. Stone S, Lin W. The unfolded protein response in multiple sclerosis. Front Neurosci. 2015; 9:264.
Article25. Harada M, Nose E, Takahashi N, et al. Evidence of the activation of unfolded protein response in granulosa and cumulus cells during follicular growth and maturation. Gynecol Endocrinol. 2015; 31:783–7.
Article26. Vieira FG, Ping Q, Moreno AJ, et al. Guanabenz treatment accelerates disease in a mutant SOD1 mouse model of ALS. PLoS One. 2015; 10:e0135570.
Article27. Benmerzouga I, Checkley LA, Ferdig MT, Arrizabalaga G, Wek RC, Sullivan WJ Jr. Guanabenz repurposed as an antiparasitic with activity against acute and latent toxoplasmosis. Antimicrob Agents Chemother. 2015; 59:6939–45.
Article28. Hamamura K, Minami K, Tanjung N, et al. Attenuation of malignant phenotypes of breast cancer cells through eIF2alpha-mediated downregulation of Rac1 signaling. Int J Oncol. 2014; 44:1980–8.29. Jadamba E, Shin M. A systematic framework for drug repositioning from integrated omics and drug phenotype profiles using pathway-drug network. Biomed Res Int. 2016; 2016:7147039.
Article30. Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004; 3:673–83.
Article31. Temesi G, Bolgár B, Arany A, Szalai C, Antal P, Mátyus P. Early repositioning through compound set enrichment analysis: a knowledgerecycling strategy. Future Med Chem. 2014; 6:563–75.
Article32. Mizushima T. Identification of a molecular mechanism for actions of existing medicines and its application for drug development. Yakugaku Zasshi. 2012; 132:713–20.33. Schwab C, Jagannath S. The role of thalidomide in multiple myeloma. Clin Lymphoma Myeloma. 2006; 7:26–9.
Article34. Walker SL, Waters MF, Lockwood DN. The role of thalidomide in the management of erythema nodosum leprosum. Lepr Rev. 2007; 78:197–215.
Article35. Choulier L, Nomine Y, Zeder-Lutz G, et al. Chemical library screening using a SPR-based inhibition in solution assay: simulations and experimental validation. Anal Chem. 2013; 85:8787–95.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoplasmic Reticulum Stress and Diabetes
- Blockade of Autophagy Aggravates Endoplasmic Reticulum Stress and Improves Paclitaxel Cytotoxicity in Human Cervical Cancer Cells
- Endoplasmic Reticulum Stress Responses and Apoptosis
- Celecoxib induces cell death on non-small cell lung cancer cells through endoplasmic reticulum stress
- Endoplasmic Reticulum Stress and Dysregulated Autophagy in Human Pancreatic Beta Cells