Anat Cell Biol.
2017 Dec;50(4):293-300. 10.5115/acb.2017.50.4.293.
Celecoxib induces cell death on non-small cell lung cancer cells through endoplasmic reticulum stress
- Affiliations
-
- 1Department of Pathology, Inje University Haeundae Paik Hospital, Busan, Korea.
- 2Department of Anatomy and Research Center for Tumor Immunology, Inje University College of Medicine, Busan, Korea. newsoft@inje.ac.kr
- KMID: 2399898
- DOI: http://doi.org/10.5115/acb.2017.50.4.293
Abstract
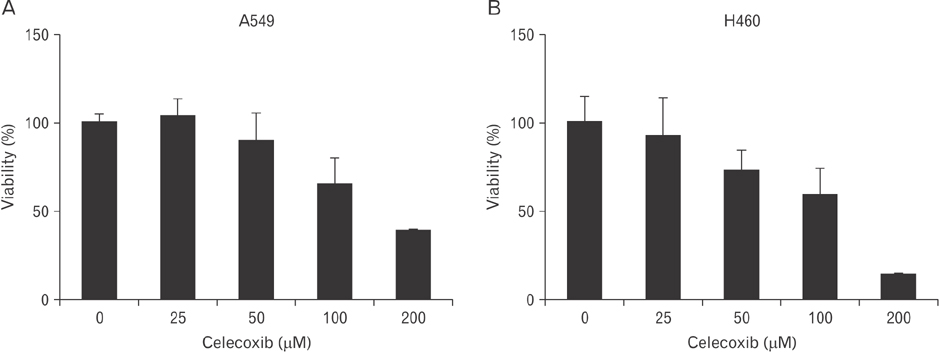
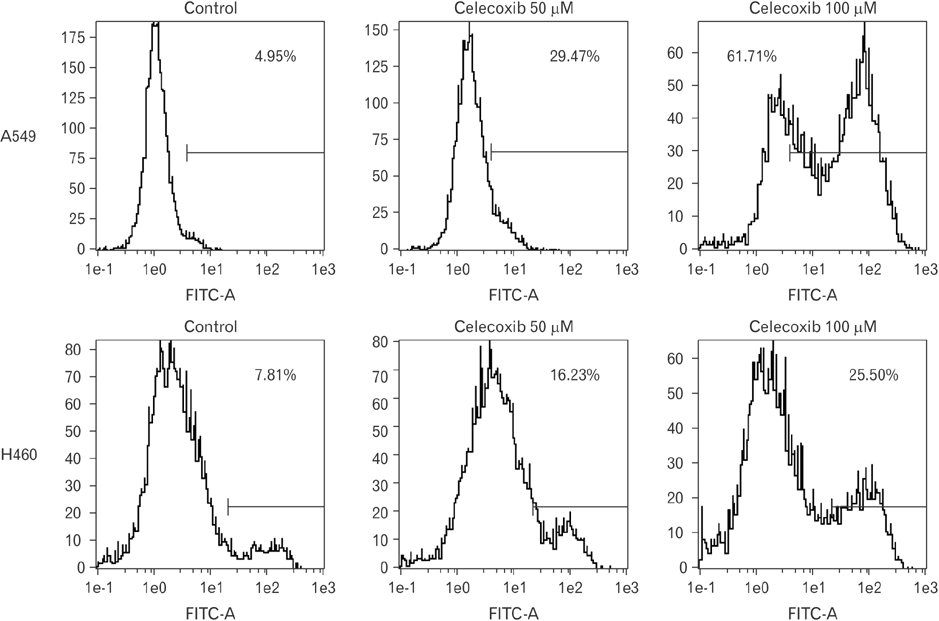
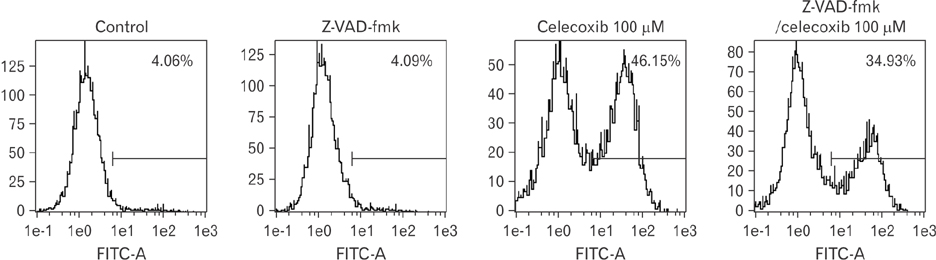
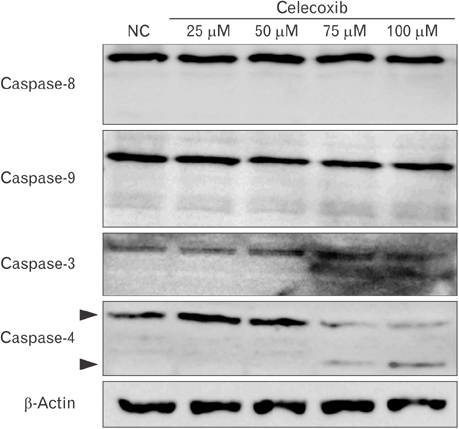
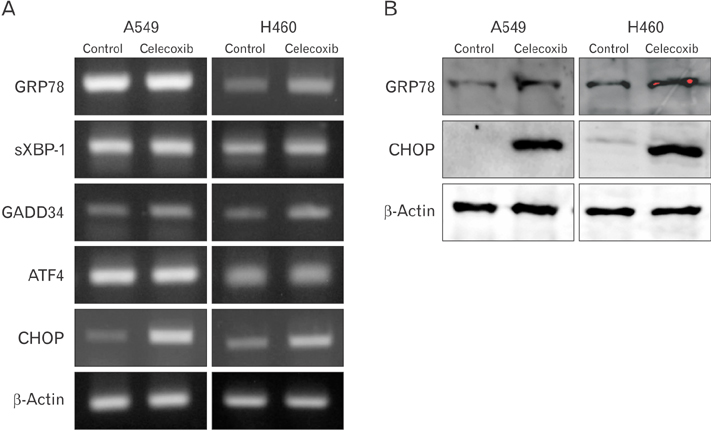
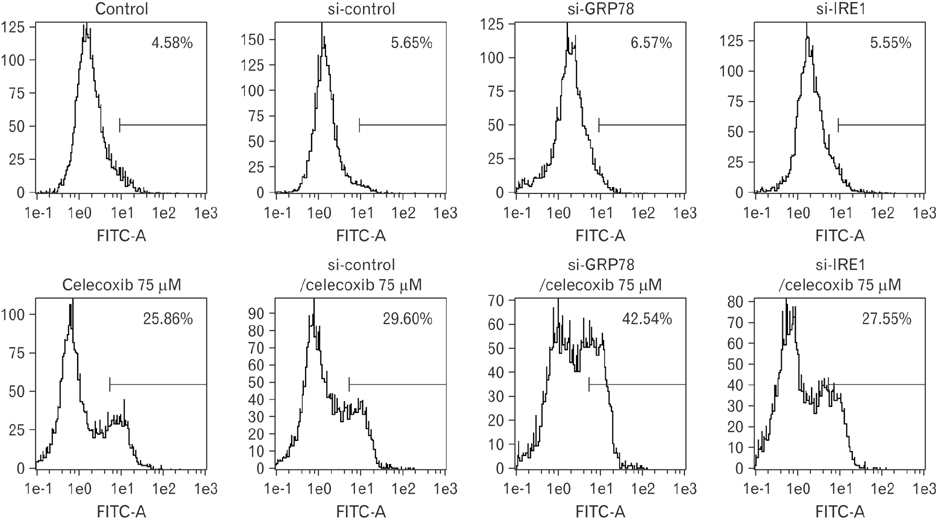
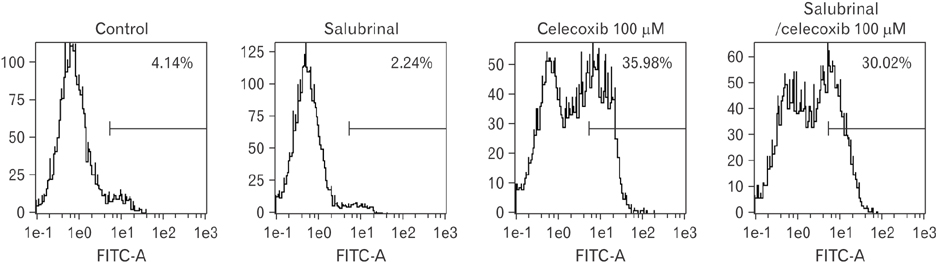
- Cyclooxygenase-2 (COX-2) is an enzyme induced by various proinflammatory and mitogenic stimuli. Celecoxib is a selective inhibitor of COX-2 that have been shown to affect cell growth and apoptosis. Lung cancer cells expressing COX-2 is able to be a target of celecoxib, this study focuses on investigating that celecoxib induces apoptosis via endoplasmic reticulum (ER) stress on lung cancer cells. We investigated whether celecoxib induced apoptosis on non-small cell lung cancer cell line, A549 and H460. The 50 µM of celecoxib increased apoptotic cells and 100 µM of celecoxib significantly induced apoptosis. To check involvement of caspase cascade, pretreatment of z-VAD-fmk blocked celecoxib-induced apoptosis. However, caspase-3, -8, and -9 were not activated, but cleavage of non-classical caspase-4 was detected using western blot. As checking ER stress associated molecules, celecoxib did not increase expressions of growth arrest and DNA damage inducible protein 34, activating transcription factor 4, and spliced X-box binding protiens-1, but increase of both glucose-regulated protein 78 (GRP78) and C/EBP homologous transcription factor were detected. Salubrinal, inhibitor of eIF2 and siRNA for IRE1 did not alter celecoxib-induced apoptosis. Instead, celecoxib-induced apoptosis might be deeply associated with ER stress depending on GRP78 because siRNA for GRP78 enhanced apoptosis. Taken together, celecoxib triggered ER stress on lung cancer cells and celecoxib-induced apoptosis might be involved in both non-classical caspase-4 and GRP78.
Keyword
MeSH Terms
-
Activating Transcription Factor 4
Apoptosis
Blotting, Western
Carcinoma, Non-Small-Cell Lung*
Caspase 3
Celecoxib*
Cell Death*
Cell Line
Cyclooxygenase 2
DNA Damage
Endoplasmic Reticulum Stress*
Endoplasmic Reticulum*
Eukaryotic Initiation Factor-2
Lung Neoplasms
RNA, Small Interfering
Transcription Factors
Activating Transcription Factor 4
Caspase 3
Celecoxib
Cyclooxygenase 2
Eukaryotic Initiation Factor-2
RNA, Small Interfering
Transcription Factors
Figure
Reference
-
1. Hirsch FR, Lippman SM. Advances in the biology of lung cancer chemoprevention. J Clin Oncol. 2005; 23:3186–3197.2. Keith RL. Lung cancer chemoprevention. Proc Am Thorac Soc. 2012; 9:52–56.3. Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011; 32:605–644.4. Sandler AB, Dubinett SM. COX-2 inhibition and lung cancer. Semin Oncol. 2004; 31:2 Suppl 7. 45–52.5. Salgia R, Skarin AT. Molecular abnormalities in lung cancer. J Clin Oncol. 1998; 16:1207–1217.6. Collins LG, Haines C, Perkel R, Enck RE. Lung cancer: diagnosis and management. Am Fam Physician. 2007; 75:56–63.7. Dy GK, Adjei AA. Novel targets for lung cancer therapy: part I. J Clin Oncol. 2002; 20:2881–2894.8. Gasparini G, Longo R, Sarmiento R, Morabito A. Inhibitors of cyclo-oxygenase 2: a new class of anticancer agents? Lancet Oncol. 2003; 4:605–615.9. Breyer MD, Harris RC. Cyclooxygenase 2 and the kidney. Curr Opin Nephrol Hypertens. 2001; 10:89–98.10. Frungieri MB, Calandra RS, Mayerhofer A, Matzkin ME. Cyclooxygenase and prostaglandins in somatic cell populations of the testis. Reproduction. 2015; 149:R169–R180.11. Vosooghi M, Amini M. The discovery and development of cyclooxygenase-2 inhibitors as potential anticancer therapies. Expert Opin Drug Discov. 2014; 9:255–267.12. Ghosh N, Chaki R, Mandal V, Mandal SC. COX-2 as a target for cancer chemotherapy. Pharmacol Rep. 2010; 62:233–244.13. Rizzo MT. Cyclooxygenase-2 in oncogenesis. Clin Chim Acta. 2011; 412:671–687.14. Grösch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst. 2006; 98:736–747.15. Kismet K, Akay MT, Abbasoglu O, Ercan A. Celecoxib: a potent cyclooxygenase-2 inhibitor in cancer prevention. Cancer Detect Prev. 2004; 28:127–142.16. Jendrossek V. Targeting apoptosis pathways by celecoxib in cancer. Cancer Lett. 2013; 332:313–324.17. Ko SH, Choi GJ, Lee JH, Han YA, Lim SJ, Kim SH. Differential effects of selective cyclooxygenase-2 inhibitors in inhibiting proliferation and induction of apoptosis in oral squamous cell carcinoma. Oncol Rep. 2008; 19:425–433.18. Wu T, Leng J, Han C, Demetris AJ. The cyclooxygenase-2 inhibitor celecoxib blocks phosphorylation of Akt and induces apoptosis in human cholangiocarcinoma cells. Mol Cancer Ther. 2004; 3:299–307.19. Liggett JL, Zhang X, Eling TE, Baek SJ. Anti-tumor activity of non-steroidal anti-inflammatory drugs: cyclooxygenase-independent targets. Cancer Lett. 2014; 346:217–224.20. Wang WA, Groenendyk J, Michalak M. Endoplasmic reticulum stress associated responses in cancer. Biochim Biophys Acta. 2014; 1843:2143–2149.21. Chevet E, Hetz C, Samali A. Endoplasmic reticulum stress-activated cell reprogramming in oncogenesis. Cancer Discov. 2015; 5:586–597.22. Huang KH, Kuo KL, Chen SC, Weng TI, Chuang YT, Tsai YC, Pu YS, Chiang CK, Liu SH. Down-regulation of glucose-regulated protein (GRP) 78 potentiates cytotoxic effect of celecoxib in human urothelial carcinoma cells. PLoS One. 2012; 7:e33615.23. Tsutsumi S, Namba T, Tanaka KI, Arai Y, Ishihara T, Aburaya M, Mima S, Hoshino T, Mizushima T. Celecoxib upregulates endoplasmic reticulum chaperones that inhibit celecoxib-induced apoptosis in human gastric cells. Oncogene. 2006; 25:1018–1029.24. Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013; 1833:3460–3470.25. McGuckin MA, Eri RD, Das I, Lourie R, Florin TH. ER stress and the unfolded protein response in intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2010; 298:G820–G832.26. Tsutsumi S, Gotoh T, Tomisato W, Mima S, Hoshino T, Hwang HJ, Takenaka H, Tsuchiya T, Mori M, Mizushima T. Endoplasmic reticulum stress response is involved in nonsteroidal anti-inflammatory drug-induced apoptosis. Cell Death Differ. 2004; 11:1009–1016.27. Zhou XM, Wong BC, Fan XM, Zhang HB, Lin MC, Kung HF, Fan DM, Lam SK. Non-steroidal anti-inflammatory drugs induce apoptosis in gastric cancer cells through up-regulation of bax and bak. Carcinogenesis. 2001; 22:1393–1397.28. Duncan K, Uwimpuhwe H, Czibere A, Sarkar D, Libermann TA, Fisher PB, Zerbini LF. NSAIDs induce apoptosis in nonproliferating ovarian cancer cells and inhibit tumor growth in vivo. IUBMB Life. 2012; 64:636–643.29. Li M, Wu X, Xu XC. Induction of apoptosis in colon cancer cells by cyclooxygenase-2 inhibitor NS398 through a cytochrome c-dependent pathway. Clin Cancer Res. 2001; 7:1010–1016.30. Ramer R, Walther U, Borchert P, Laufer S, Linnebacher M, Hinz B. Induction but not inhibition of COX-2 confers human lung cancer cell apoptosis by celecoxib. J Lipid Res. 2013; 54:3116–3129.31. Gentz SH, Bertollo CM, Souza-Fagundes EM, da Silva AM. Implication of eIF2alpha kinase GCN2 in induction of apoptosis and endoplasmic reticulum stress-responsive genes by sodium salicylate. J Pharm Pharmacol. 2013; 65:430–440.32. Chen ST, Thomas S, Gaffney KJ, Louie SG, Petasis NA, Schönthal AH. Cytotoxic effects of celecoxib on Raji lymphoma cells correlate with aggravated endoplasmic reticulum stress but not with inhibition of cyclooxygenase-2. Leuk Res. 2010; 34:250–253.33. Mandegary A, Torshabi M, Seyedabadi M, Amirheidari B, Sharif E, Ghahremani MH. Indomethacin-enhanced anticancer effect of arsenic trioxide in A549 cell line: involvement of apoptosis and phospho-ERK and p38 MAPK pathways. Biomed Res Int. 2013; 2013:237543.34. Liu X, Yue P, Zhou Z, Khuri FR, Sun SY. Death receptor regulation and celecoxib-induced apoptosis in human lung cancer cells. J Natl Cancer Inst. 2004; 96:1769–1780.35. Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, Tsujimoto Y, Tohyama M. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol. 2004; 165:347–356.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Blockade of Autophagy Aggravates Endoplasmic Reticulum Stress and Improves Paclitaxel Cytotoxicity in Human Cervical Cancer Cells
- Endoplasmic Reticulum Stress Responses and Apoptosis
- Erratum: Toxoplasma gondii Induces Apoptosis via Endoplasmic Reticulum Stress-Derived Mitochondrial Pathway in Human Small Intestinal Epithelial Cell-Line
- Endoplasmic Reticulum Stress and Diabetes
- Endoplasmic Reticulum Stress and Dysregulated Autophagy in Human Pancreatic Beta Cells