Cancer Res Treat.
2015 Apr;47(2):313-321. 10.4143/crt.2013.222.
Blockade of Autophagy Aggravates Endoplasmic Reticulum Stress and Improves Paclitaxel Cytotoxicity in Human Cervical Cancer Cells
- Affiliations
-
- 1Department of Respiratory Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China.
- 2Department of Obstetrics and Gynecology, The Shanxi Da Yi Hospital, Taiyuan, China. wangzanhong@126.com
- KMID: 2132811
- DOI: http://doi.org/10.4143/crt.2013.222
Abstract
- PURPOSE
Autophagy is one of the ways to degrade unfolded proteins after endoplasmic reticulum (ER) stress. The purpose of this study is to determine whether a blockade of autophagy leads to aggravated endoplasmic reticulum stress, which then induces cells apoptosis in HeLa cells treated with paclitaxel.
MATERIALS AND METHODS
Autophagy activation and the proapoptotic effects were characterized using monodansylcadaverine labeling and Hoechest staining, respectively. A Western blot analysis was used to detect the expression of apoptotic and autophagy-related genes. A flow cytometry was used to assess the cell apoptosis ratio.
RESULTS
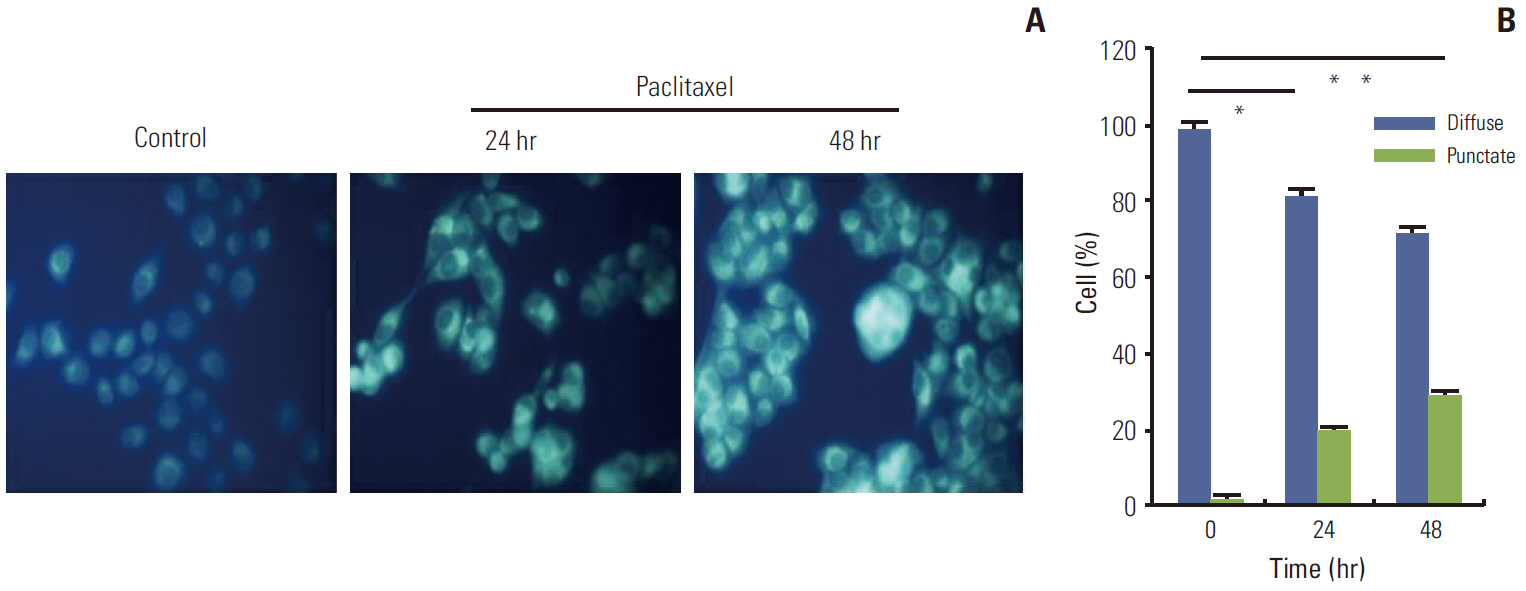
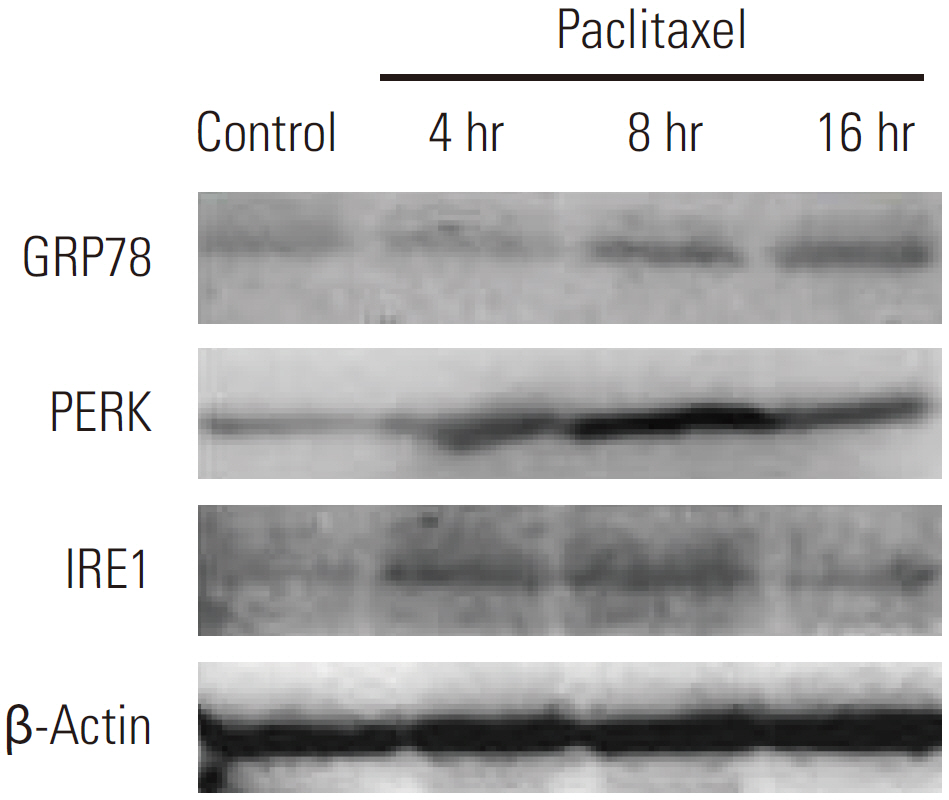
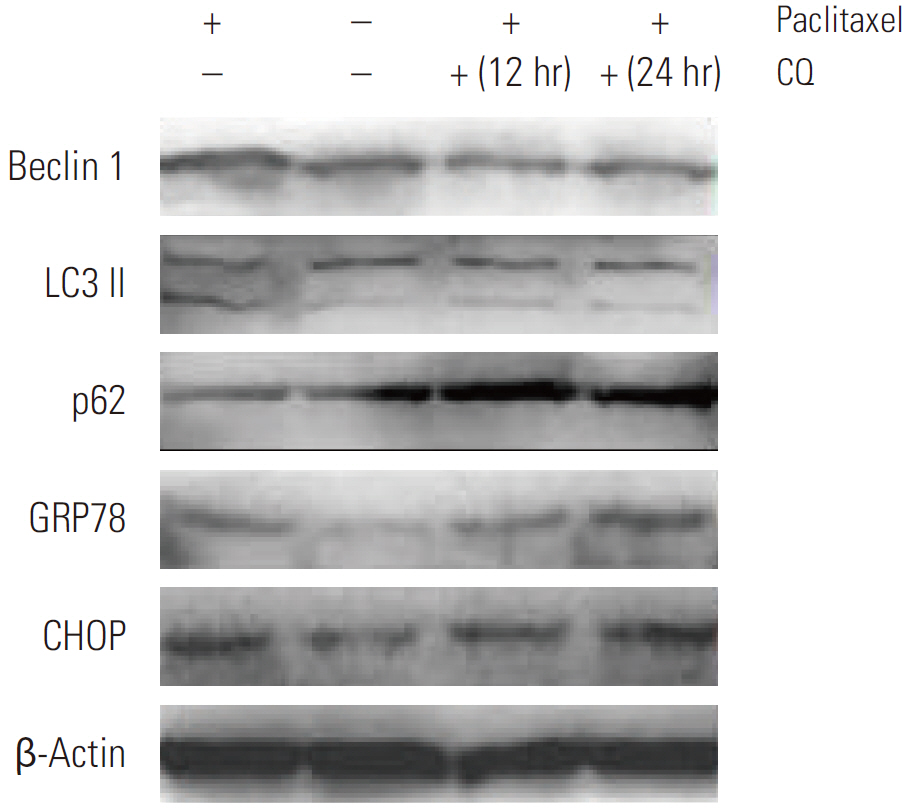
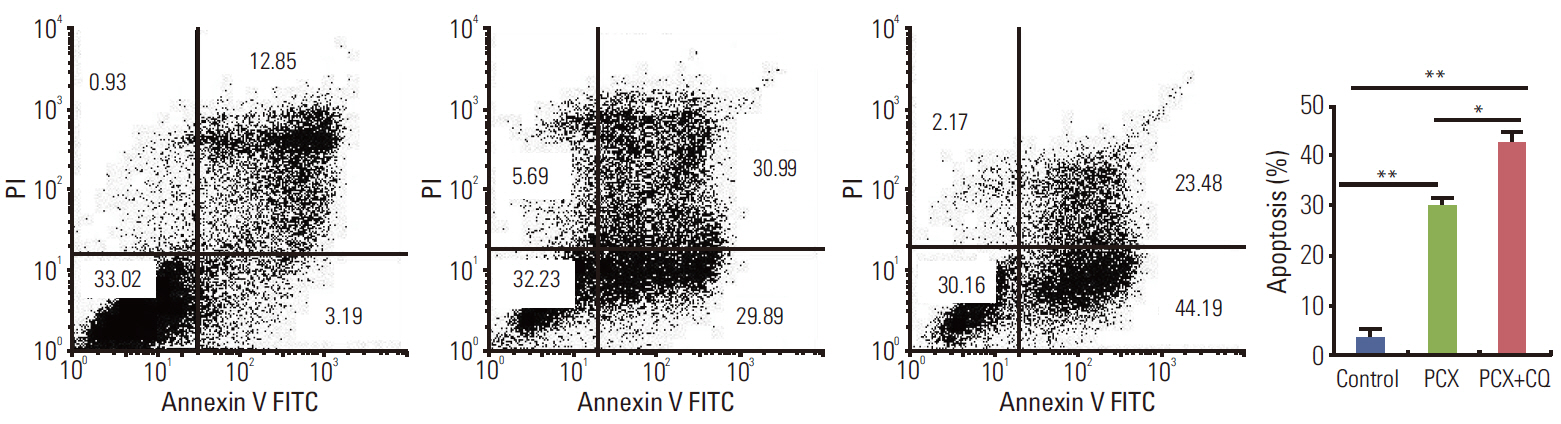
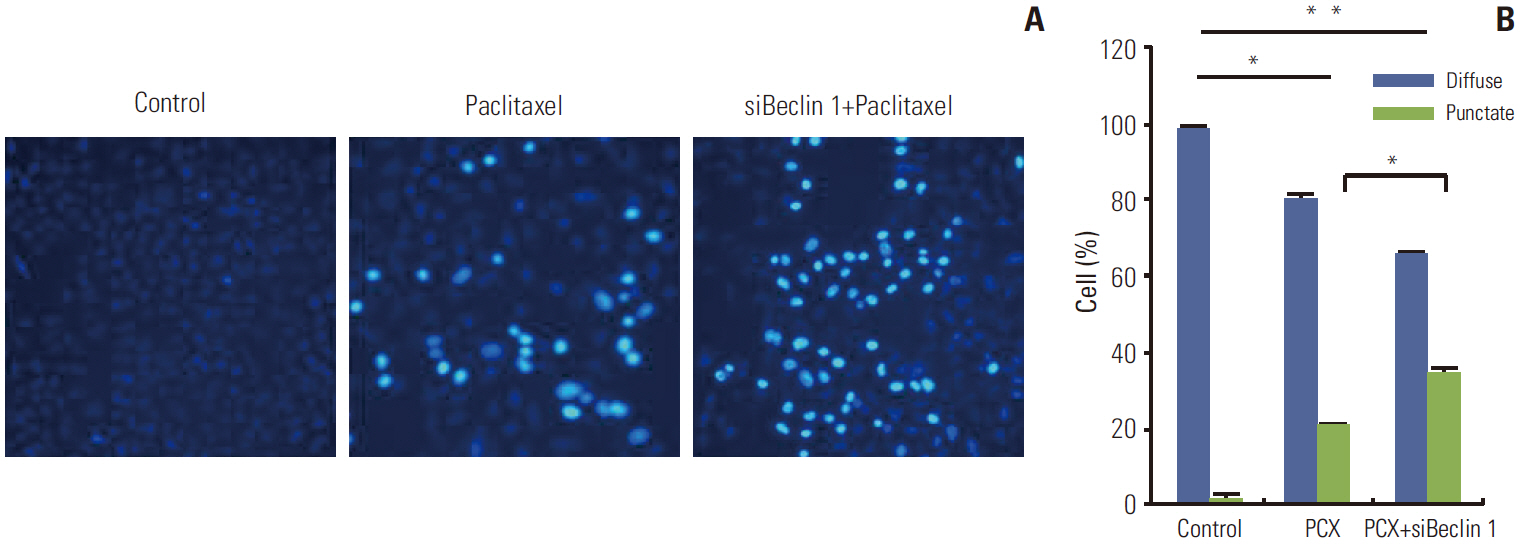
Paclitaxel exposure induced the aggregation of autophagosomes in the cytoplasms of cervical cancer HeLa cells. The expression of Beclin 1 and LC3 II were upregulated, but p62 was downregulated, which suggests that autophagy was promoted by paclitaxel. On the other hand, the expression of GRP78 obviously increased, suggesting that ER stress was induced after paclitaxel treatment. The cell proliferation assay indicated that a knockdown of Beclin 1 sensitized HeLa cells to paclitaxel. Furthermore, paclitaxel-mediated apoptotic cell death was further potentiated by the pretreatment with autophagy inhibitor chloroquine or small interfering RNA against Beclin 1. These results suggest that an induction of autophagy by paclitaxel may induce cell survival rather than cell death in HeLa cells; moreover, inhibition of autophagy led to an aggravated ER stress and an induction of downstream apoptosis.
CONCLUSION
Our results reveal autophagy induced by paclitaxel conferred protection of tumor cells against apoptosis, and blockade of autophagy subsequently aggravated ER stress, enhancing the apoptosis associated with paclitaxel treatment in HeLa cells.
MeSH Terms
Figure
Reference
-
References
1. Eum KH, Lee M. Crosstalk between autophagy and apoptosis in the regulation of paclitaxel-induced cell death in v-Haras-transformed fibroblasts. Mol Cell Biochem. 2011; 348:61–8.
Article2. Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008; 4:600–6.
Article3. Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, et al. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007; 14:230–9.4. Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006; 26:9220–31.5. Xu Y, Yu H, Qin H, Kang J, Yu C, Zhong J, et al. Inhibition of autophagy enhances cisplatin cytotoxicity through endoplasmic reticulum stress in human cervical cancer cells. Cancer Lett. 2012; 314:232–43.
Article6. Suh DH, Kim JW, Kim K, Kim HJ, Lee KH. Major clinical research advances in gynecologic cancer in 2012. J Gynecol Oncol. 2013; 24:66–82.
Article7. Sun Y, Liu JH, Jin L, Lin SM, Yang Y, Sui YX, et al. Overexpression of the Beclin1 gene upregulates chemosensitivity to anti-cancer drugs by enhancing therapy-induced apoptosis in cervix squamous carcinoma CaSki cells. Cancer Lett. 2010; 294:204–10.
Article8. Veldhoen RA, Banman SL, Hemmerling DR, Odsen R, Simmen T, Simmonds AJ, et al. The chemotherapeutic agent paclitaxel inhibits autophagy through two distinct mechanisms that regulate apoptosis. Oncogene. 2013; 32:736–46.
Article9. Jordan MA, Toso RJ, Thrower D, Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci U S A. 1993; 90:9552–6.
Article10. Shi J, Orth JD, Mitchison T. Cell type variation in responses to antimitotic drugs that target microtubules and kinesin-5. Cancer Res. 2008; 68:3269–76.
Article11. Gorka M, Daniewski WM, Gajkowska B, Lusakowska E, Godlewski MM, Motyl T. Autophagy is the dominant type of programmed cell death in breast cancer MCF-7 cells exposed to AGS 115 and EFDAC, new sesquiterpene analogs of paclitaxel. Anticancer Drugs. 2005; 16:777–88.12. Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006; 8:1391–418.13. Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008; 7:1013–30.
Article14. Kandala PK, Srivastava SK. Regulation of macroautophagy in ovarian cancer cells in vitro and in vivo by controlling glucose regulatory protein 78 and AMPK. Oncotarget. 2012; 3:435–49.
Article15. Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006; 7:880–5.
Article16. Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010; 40:280–93.
Article17. Hoyer-Hansen M, Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007; 14:1576–82.
Article18. Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005; 25:1025–40.
Article19. Sridhar S, Botbol Y, Macian F, Cuervo AM. Autophagy and disease: always two sides to a problem. J Pathol. 2012; 226:255–73.
Article20. Carew JS, Nawrocki ST, Cleveland JL. Modulating autophagy for therapeutic benefit. Autophagy. 2007; 3:464–7.
Article21. Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007; 117:326–36.
Article22. Xi G, Hu X, Wu B, Jiang H, Young CY, Pang Y, et al. Autophagy inhibition promotes paclitaxel-induced apoptosis in cancer cells. Cancer Lett. 2011; 307:141–8.
Article23. Kim HJ, Lee SG, Kim YJ, Park JE, Lee KY, Yoo YH, et al. Cytoprotective role of autophagy during paclitaxel-induced apoptosis in Saos-2 osteosarcoma cells. Int J Oncol. 2013; 42:1985–92.
Article24. Liao PC, Tan SK, Lieu CH, Jung HK. Involvement of endoplasmic reticulum in paclitaxel-induced apoptosis. J Cell Biochem. 2008; 104:1509–23.
Article25. Wu Y, Fabritius M, Ip C. Chemotherapeutic sensitization by endoplasmic reticulum stress: increasing the efficacy of taxane against prostate cancer. Cancer Biol Ther. 2009; 8:146–52.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoplasmic Reticulum Stress and Dysregulated Autophagy in Human Pancreatic Beta Cells
- Endoplasmic Reticulum Stress and Diabetes
- Role of autophagy in diabetes and endoplasmic reticulum stress of pancreatic beta-cells
- New Insights into the Role of Endoplasmic Reticulum Stress in Breast Cancer Metastasis
- Autophagy in Diabetes