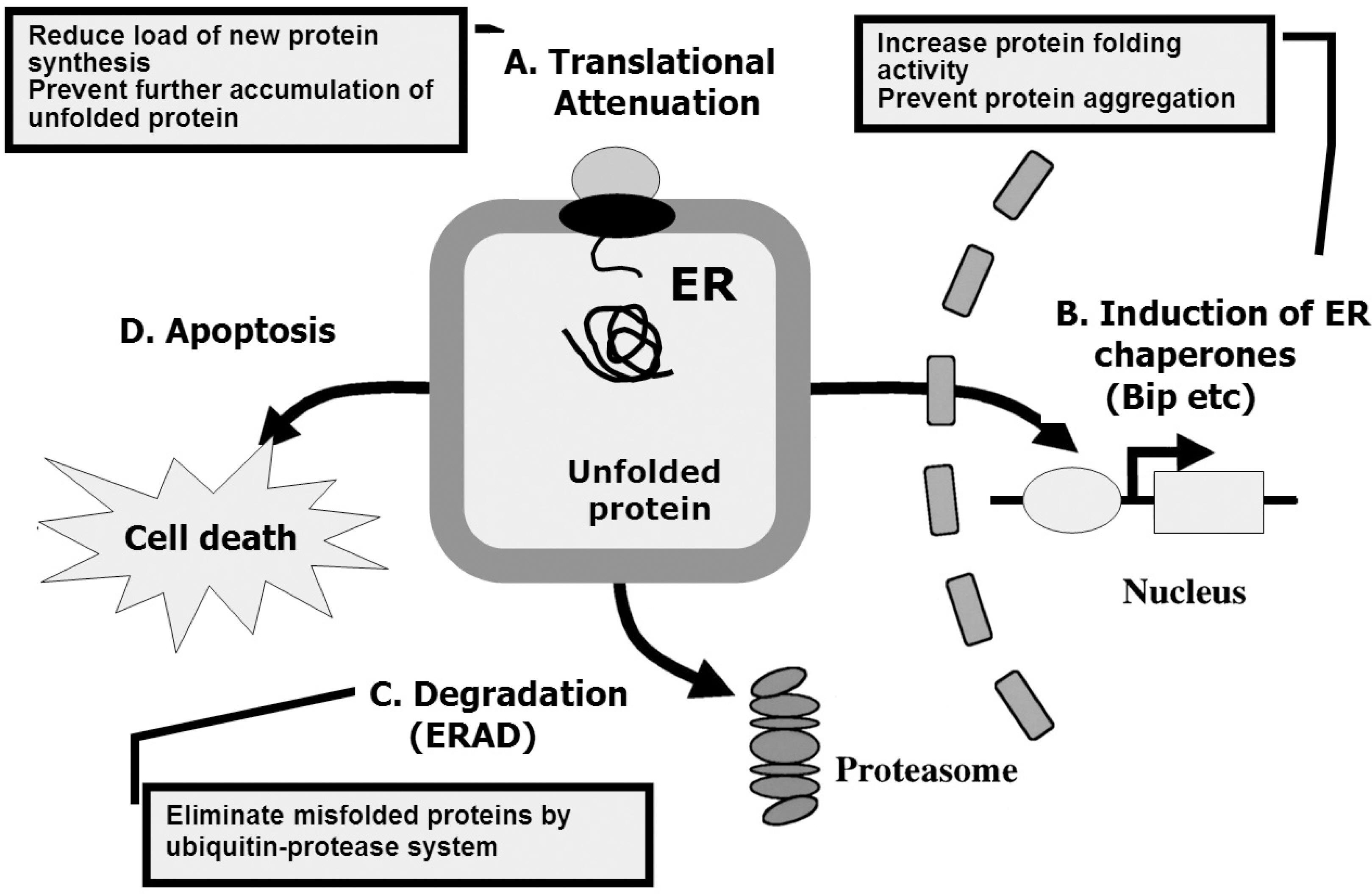
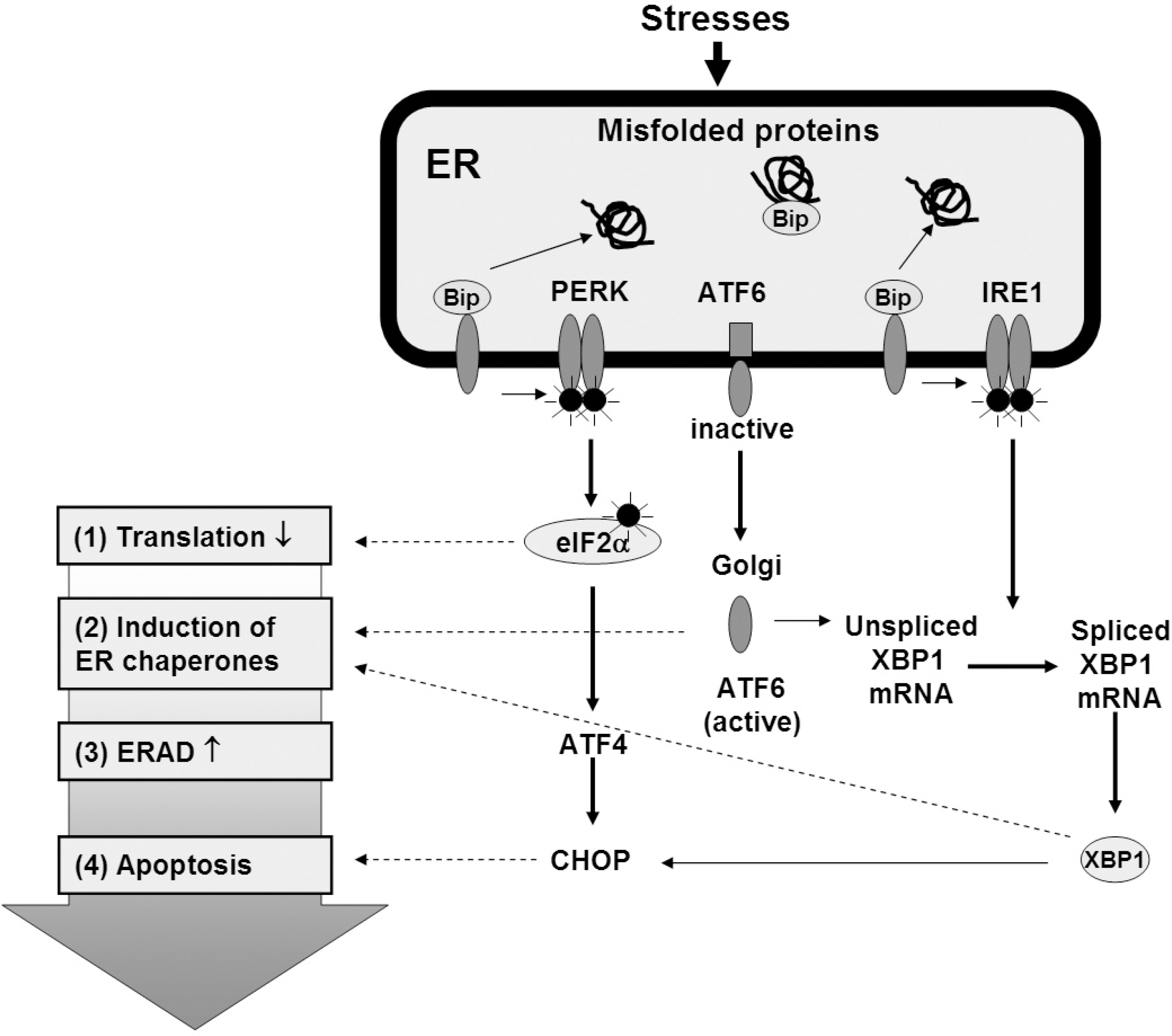
J Korean Endocr Soc.
2008 Feb;23(1):1-8. 10.3803/jkes.2008.23.1.1.
Endoplasmic Reticulum Stress and Diabetes
- Affiliations
-
- 1Department of Internal Medicine, Keimyung University School of Medicine, Korea.
- KMID: 2063543
- DOI: http://doi.org/10.3803/jkes.2008.23.1.1
Abstract
- No abstract available.
Figure
Cited by 1 articles
-
Chronic Alcohol Consumption Results in Greater Damage to the Pancreas Than to the Liver in the Rats
Seong-Su Lee, Oak-Kee Hong, Anes Ju, Myung-Jun Kim, Bong-Jo Kim, Sung-Rae Kim, Won-Ho Kim, Nam-Han Cho, Moo-Il Kang, Sung-Koo Kang, Dai-Jin Kim, Soon-Jib Yoo
Korean J Physiol Pharmacol. 2015;19(4):309-318. doi: 10.4196/kjpp.2015.19.4.309.
Reference
-
References
1. Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 13:1211–1233. 1999.
Article2. Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 74:739–789. 2005.
Article3. Oyadomari S, Araki E, Mori M. Endoplasmic reticulum stress mediated apoptosis in pancreatic β-cell. Apoptosis. 7:335–345. 2002.4. Mori K. Tripartitie management of unfolded proteins in the endoplasmic reticulum. Cell. 101:451–454. 2000.5. Kaufman RJ, Scheuner D, Schroder M, Shen X, Lee K, Liu CY, Arnold SM. The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol. 3:411–421. 2002.
Article6. Harding HP, Calfon M, Urano F, Novoa I and Ron D. Transcriptional and translational control in the mammalian unfolded protein response. Annu Rev Cell Dev Biol. 18:575–599. 2002.
Article7. Kozutsumi MY, Segal M, Normington K, Gething MJ and Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 332:462–464. 1988.
Article8. Yoshida H, Haze K, Yanagi H, Yura T and Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem. 273:33741–33749. 1998.9. Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS and Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 101:249–258. 2000.
Article10. Araki E, Oyadomari S, Mori M. Impact of endoplasmic reticulum stress pathway on pancreatic β-cells and diabetes mellitus. Exp Biol Med. 228:1213–1217. 2003.
Article11. Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 397:271–274. 1999.
Article12. Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in Perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell. 7:1153–1163. 2001.
Article13. Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 6:1099–1108. 2000.
Article14. Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 415:92–96. 2002.
Article15. Hai TW, Liu F, Coukos WJ, Green MR. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 3:2083–2090. 1989.
Article16. Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 10:3787–3799. 1999.
Article17. Chen X, Shen J, Prywes R. The luminal domain of ATF6 senses endoplasmic reticulum(ER) stress and causes translocation of ATF6 from the ER to the Golgi. J Biol Chem. 277:13045–13052. 2002.18. Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL. ER stress induces cleavage of membrane bound ATF6 by the same proteases that process SREBPs. Mol Cell. 6:1355–1364. 2000.19. Wang Y, Shen J, Arenzana N, Tirasophon W, Kaufman RJ, Prywes R. Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J Biol Chem. 275:27013–27020. 2000.
Article20. Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K. A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell. 4:265–271. 2003.
Article21. Wiertz EJ, Tortorella D, Bogyo M, Yu J, Mothes W, Jones TR, Rapoport TA, Ploegh HL. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 384:432–438. 1996.22. Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell surival during the unfolded protein response. Mol Cell. 5:897–904. 2000.23. Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K. ATF6 activated by proteolysis binds in the presence of NF-Y(CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol. 20:6755–6767. 2000.24. Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress response: EMBO. 19:5708–5717. 1998.25. Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 12:982–995. 1998.26. McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 21:1249–1259. 2001.
Article27. Oydomari S, Takeda K Takiguchi M, Gotoh T, Matsumoto M, Wada I, Akira S, Araki E, Mori M. Nitric oxide-induced apoptosis in pancreatic β cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci USA. 98:10845–10850. 2001.28. Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yanker BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 406:98–103. 2000.29. Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 287:664–666. 2000.
Article30. Yoshioka M, Kayo T, Ikeda T, Kooizumi A. A novel locus, Mody 4 distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 46:887–894. 1997.31. Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizuimi A, Izuimi T. A mutation in the insulin 2 gene induces diabetes with severe pancreatic β-cell dysfunction in the Mody mouse. J Clin Invest. 103:27–37. 1999.
Article32. Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 109:525–532. 2002.
Article33. Delpine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-α kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 25:406–409. 2000.
Article34. Zhang W, Feng D, Li Y, Lida K, McGrath B, Cavener DR. PERK EIF2AK3 control of pancreatic β cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 4:491–497. 2006.
Article35. Scheuner D, Vander Mierde D, Song B, Flamez D, Creemers JW, Tsukamoto K, Ribick M, Schuit FC, Kaufman RJ. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 11:757–764. 2005.
Article36. Wang H, Kouri G, Wolheim CB. ER stress and SREBP-1 activation are implicated in β-cell glucolipotoxicity. J Cell Sci. 118:3905–3915. 2005.
Article37. Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 4:245–254. 2006.
Article38. Kharroubi I, Ladrière L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappa B and endoplasmic reticulum stress. Endocrinology. 145:5087–5096. 2004.39. Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 50:752–763. 2007.
Article40. Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 110:851–860. 2002.41. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 115:1111–1119. 2005.
Article42. Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 145:2273–2282. 2004.
Article43. Eizirik DL, Mandrup-Poulsen T. A choice of death-the signal transduction of immune-medicated β-cell apoptosis. Diabetologia. 44:2115–2133. 2001.44. Cardozo AK, Ortis F, Storling JS, Feng YM, Rasschaert J, Tonnesen MT, Eylen FV, Mandrup-Poulsen T, Herchuelz A, Eizirik DL. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic β-Cells. Diabetes. 54:452–461. 2005.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoplasmic Reticulum (ER) Stress and Vascular Complication
- Nuclear Receptors Resolve Endoplasmic Reticulum Stress to Improve Hepatic Insulin Resistance
- Endoplasmic Reticulum Stress Responses and Apoptosis
- Endoplasmic Reticulum Stress and Dysregulated Autophagy in Human Pancreatic Beta Cells
- New Insights into the Role of Endoplasmic Reticulum Stress in Breast Cancer Metastasis