Cancer Res Treat.
2016 Apr;48(2):738-752. 10.4143/crt.2015.102.
Anti-cancer Effect of Luminacin, a Marine Microbial Extract, in Head and Neck Squamous Cell Carcinoma Progression via Autophagic Cell Death
- Affiliations
-
- 1Department of Otolaryngology, Ajou University School of Medicine, Suwon, Korea. ostium@ajou.ac.kr
- 2Department of Molecular Science and Technology, Ajou University School of Medicine, Suwon, Korea.
- 3Natural Medicine Center, KIST Gangneung Institute, Gangneung, Korea.
- 4Department of Otorhinolaryngology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2454352
- DOI: http://doi.org/10.4143/crt.2015.102
Abstract
- PURPOSE
The purpose of this study is to determine whether luminacin, a marine microbial extract from the Streptomyces species, has anti-tumor effects on head and neck squamous cell carcinoma (HNSCC) cell lines via autophagic cell death.
MATERIALS AND METHODS
Inhibition of cell survival and increased cell death was measured using cell viability, colony forming, and apoptosis assays. Migration and invasion abilities of head and cancer cells were evaluated using wound healing, scattering, and invasion assays. Changes in the signal pathway related to autophagic cell death were investigated. Drug toxicity of luminacin was examined in in vitro HaCaT cells and an in vivo zebrafish model.
RESULTS
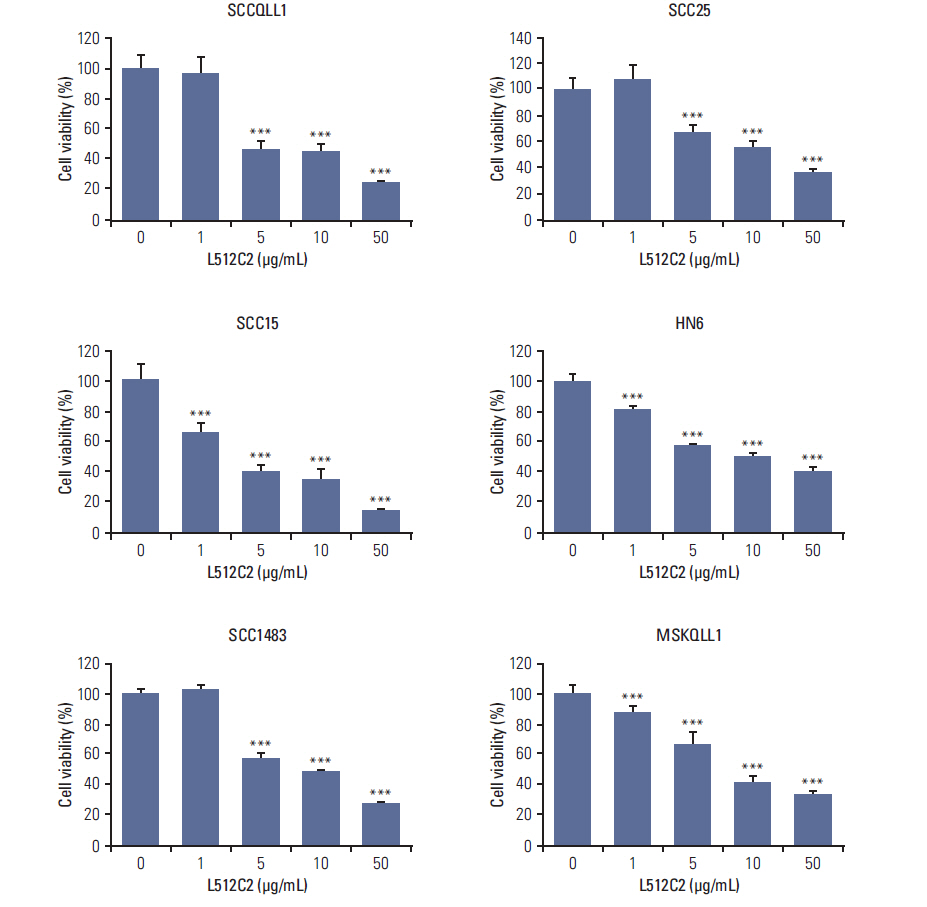
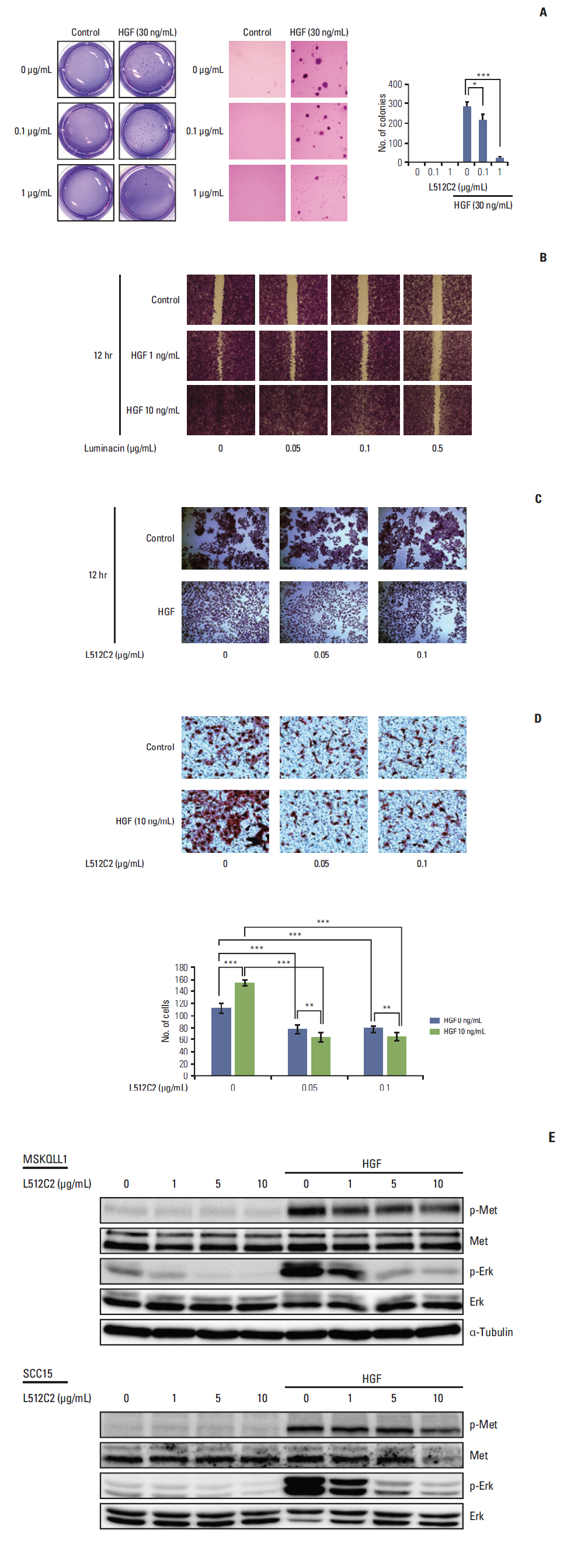
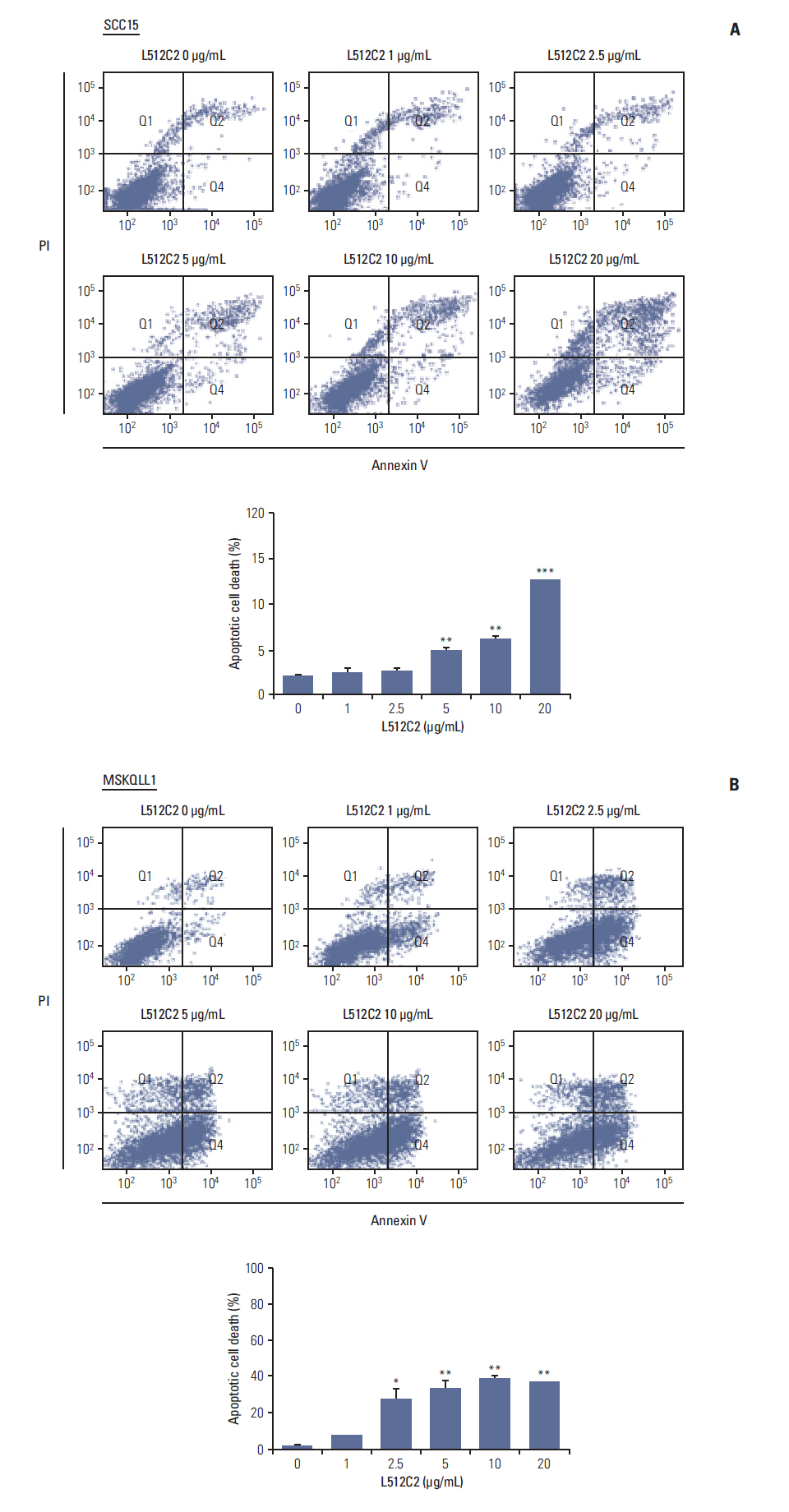
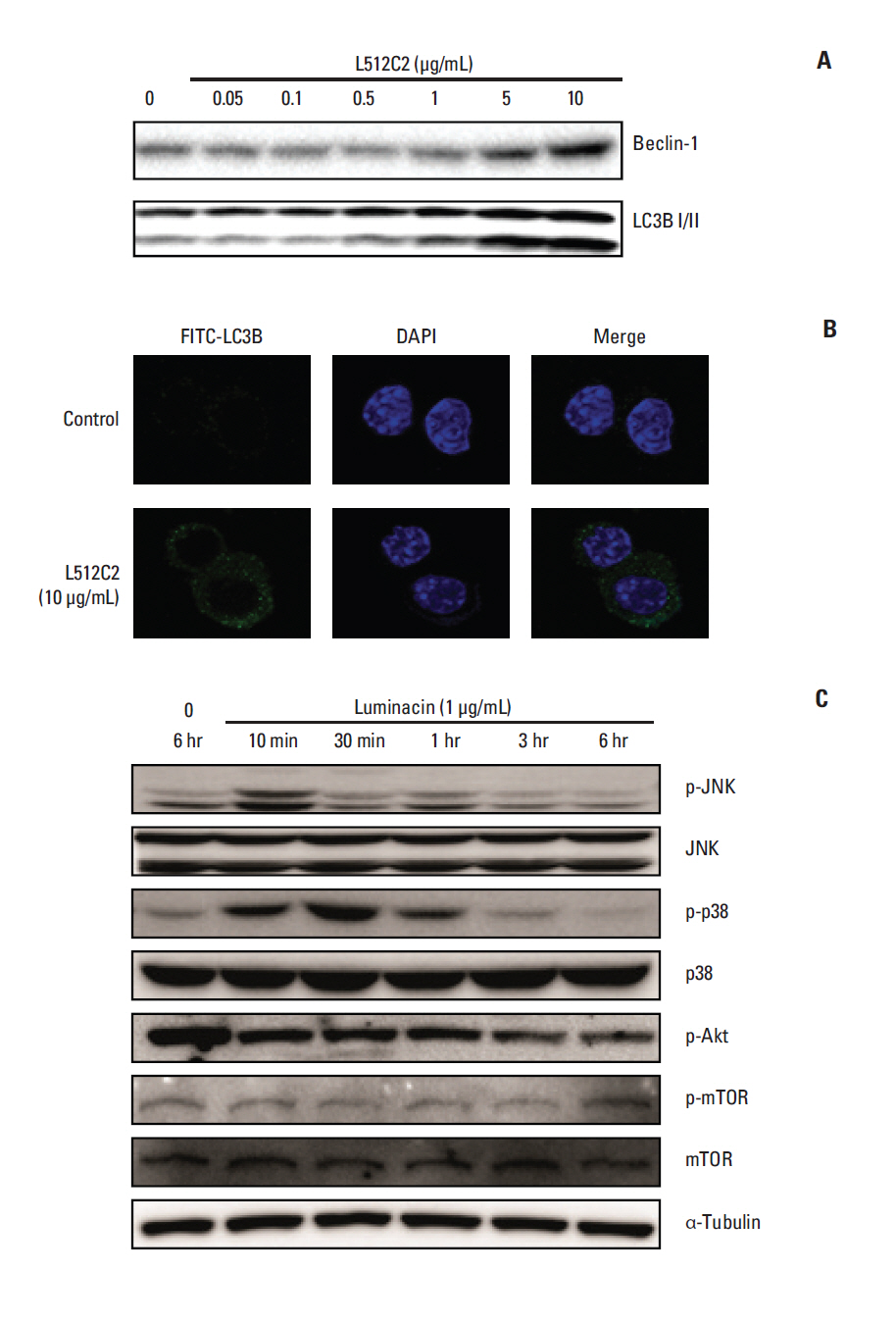
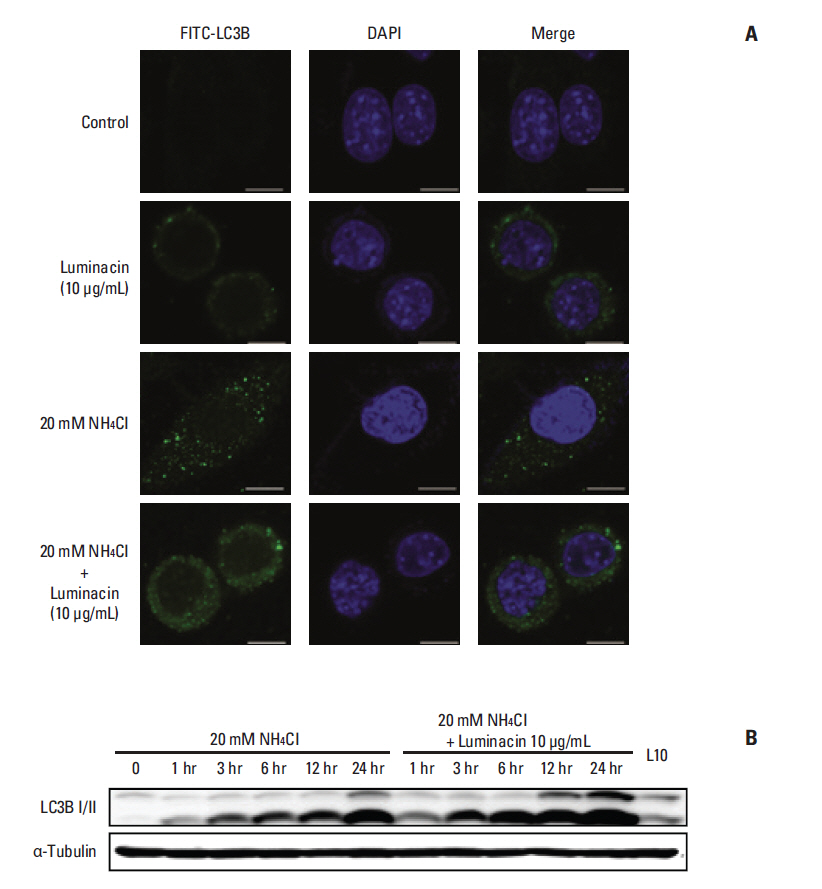
Luminacin showed potent cytotoxicity in HNSCC cells in cell viability, colony forming, and fluorescence-activated cell sorting analysis. In vitro migration and invasion of HNSCC cells were attenuated by luminacin treatment. Combined with Beclin-1 and LC3B, Luminacin induced autophagic cell death in head and neck cancer cells. In addition, in a zebrafish model and human keratinocyte cell line used for toxicity testing, luminacin treatment with a cytotoxic concentration to HNSCC cells did not cause toxicity.
CONCLUSION
Taken together, these results demonstrate that luminacin induces the inhibition of growth and cancer progression via autophagic cell death in HNSCC cell lines, indicating a possible alternative chemotherapeutic approach for treatment of HNSCC.
MeSH Terms
Figure
Reference
-
References
1. Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat. 2014; 46:109–23.
Article2. Takes RP, Rinaldo A, Silver CE, Haigentz M Jr, Woolgar JA, Triantafyllou A, et al. Distant metastases from head and neck squamous cell carcinoma. Part I. Basic aspects. Oral Oncol. 2012; 48:775–9.
Article3. Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012; 75:311–35.
Article4. Gerwick WH, Moore BS. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem Biol. 2012; 19:85–98.
Article5. Shin HA, Shin YS, Kang SU, Kim JH, Oh YT, Park KH, et al. Radioprotective effect of epicatechin in cultured human fibroblasts and zebrafish. J Radiat Res. 2014; 55:32–40.
Article6. Lee BS, Kang S, Kim KA, Song YJ, Cheong KH, Cha HY, et al. Met degradation by SAIT301, a Met monoclonal antibody, reduces the invasion and migration of nasopharyngeal cancer cells via inhibition of EGR-1 expression. Cell Death Dis. 2014; 5:e1159.
Article7. Thomas TR, Kavlekar DP, LokaBharathi PA. Marine drugs from sponge-microbe association: a review. Mar Drugs. 2010; 8:1417–68.8. Proksch P, Edrada RA, Ebel R. Drugs from the seas: current status and microbiological implications. Appl Microbiol Biotechnol. 2002; 59:125–34.9. Taylor MW, Radax R, Steger D, Wagner M. Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol Mol Biol Rev. 2007; 71:295–347.
Article10. Wang G. Diversity and biotechnological potential of the sponge-associated microbial consortia. J Ind Microbiol Biotechnol. 2006; 33:545–51.
Article11. Naruse N, Kageyama-Kawase R, Funahashi Y, Wakabayashi T. Luminacins: a family of capillary tube formation inhibitors from Streptomyces sp. I. Taxomony, fermentation, isolation, physico-chemical properties and structure elucidation. J Antibiot (Tokyo). 2000; 53:579–90.12. Wakabayashi T, Kageyama-Kawase R, Naruse N, Funahashi Y, Yoshimatsu K. Luminacins: a family of capillary tube formation inhibitors from Streptomyces sp. II. Biological activities. J Antibiot (Tokyo). 2000; 53:591–6.
Article13. Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, et al. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature. 2002; 417:141–7.
Article14. Atatreh N, Barraclough J, Welman A, Cawthorne C, Bryce RA, Dive C, et al. Difluoro analogue of UCS15A triggers activation of exogenously expressed c-Src in HCT 116 human colorectal carcinoma cells. J Enzyme Inhib Med Chem. 2007; 22:638–46.
Article15. Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004; 23:2891–906.
Article16. Li X, Wu D, Shen J, Zhou M, Lu Y. Rapamycin induces autophagy in the melanoma cell line M14 via regulation of the expression levels of Bcl-2 and Bax. Oncol Lett. 2013; 5:167–72.
Article17. Ding ZB, Hui B, Shi YH, Zhou J, Peng YF, Gu CY, et al. Autophagy activation in hepatocellular carcinoma contributes to the tolerance of oxaliplatin via reactive oxygen species modulation. Clin Cancer Res. 2011; 17:6229–38.
Article18. Shi Y, Tang B, Yu PW, Tang B, Hao YX, Lei X, et al. Autophagy protects against oxaliplatin-induced cell death via ER stress and ROS in Caco-2 cells. PLoS One. 2012; 7:e51076.
Article19. Sishi BJ, Loos B, van Rooyen J, Engelbrecht AM. Autophagy upregulation promotes survival and attenuates doxorubicin-induced cardiotoxicity. Biochem Pharmacol. 2013; 85:124–34.
Article20. Wu YT, Tan HL, Huang Q, Kim YS, Pan N, Ong WY, et al. Autophagy plays a protective role during zVAD-induced necrotic cell death. Autophagy. 2008; 4:457–66.
Article21. Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005; 4:988–1004.
Article22. Lin C, Tsai SC, Tseng MT, Peng SF, Kuo SC, Lin MW, et al. AKT serine/threonine protein kinase modulates baicalin-triggered autophagy in human bladder cancer T24 cells. Int J Oncol. 2013; 42:993–1000.
Article23. Zhou H, Shen T, Shang C, Luo Y, Liu L, Yan J, et al. Ciclopirox induces autophagy through reactive oxygen species-mediated activation of JNK signaling pathway. Oncotarget. 2014; 5:10140–50.
Article24. Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000; 103:239–52.
Article25. Zhao Z, Han F, Yang S, Wu J, Zhan W. Oxamate-mediated inhibition of lactate dehydrogenase induces protective autophagy in gastric cancer cells: involvement of the Akt-mTOR signaling pathway. Cancer Lett. 2015; 358:17–26.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Relationship between Expression of E-Cadherin, Clinical Staging and Differentiation in Squamous Cell Carcinomas of the Head and Neck
- Immunotherapy in Head and Neck Squamous Cell Cancer
- EGFR-targeted Therapy in Head and Neck Squamous Cell Carcinoma
- Herpes Viral Gene Therapy for the Treatment of Head and Neck Squamous Cell Carcinoma
- Establishment of a Cell Line (CNUH-HNSCC-1) Derived from an Advanced Laryngeal Squamous Cell Carcinoma