Int J Stem Cells.
2019 Mar;12(1):170-182. 10.15283/ijsc18051.
Effects of TGF-β1 Overexpression on Biological Characteristics of Human Dental Pulp-derived Mesenchymal Stromal Cells
- Affiliations
-
- 1Department of Pathology Laboratory Techniques, Vocational School, Beykent University, Büyükçekmece/Istanbul, Turkey. hasansalkin@beykent.edu.tr
- 2Department of Histology and Embryology, Faculty of Medicine, Erciyes University, Kayseri, Turkey.
- 3Oral and Maxillofacial Surgery, Genome and Stem Cell Center, Erciyes University, Kayseri, Turkey.
- 4Department of Histology-Embryology, Faculty of Veterinary Medicine, Erciyes University, Kayseri, Turkey.
- 5Division of Hematology, Department of Internal Medicine, Faculty of Medicine Erciyes University, Kayseri, Turkey.
- KMID: 2447235
- DOI: http://doi.org/10.15283/ijsc18051
Abstract
OBJECTIVE
The aim of our study was to investigate the effect of Transforming growth factor beta-1 (TGF-β1) gene therapy on the surface markers, multilineage differentiation, viability, apoptosis, cell cycle, DNA damage and senescence of human Dental Pulp-derived Mesenchymal Stromal Cells (hDPSC).
METHODS
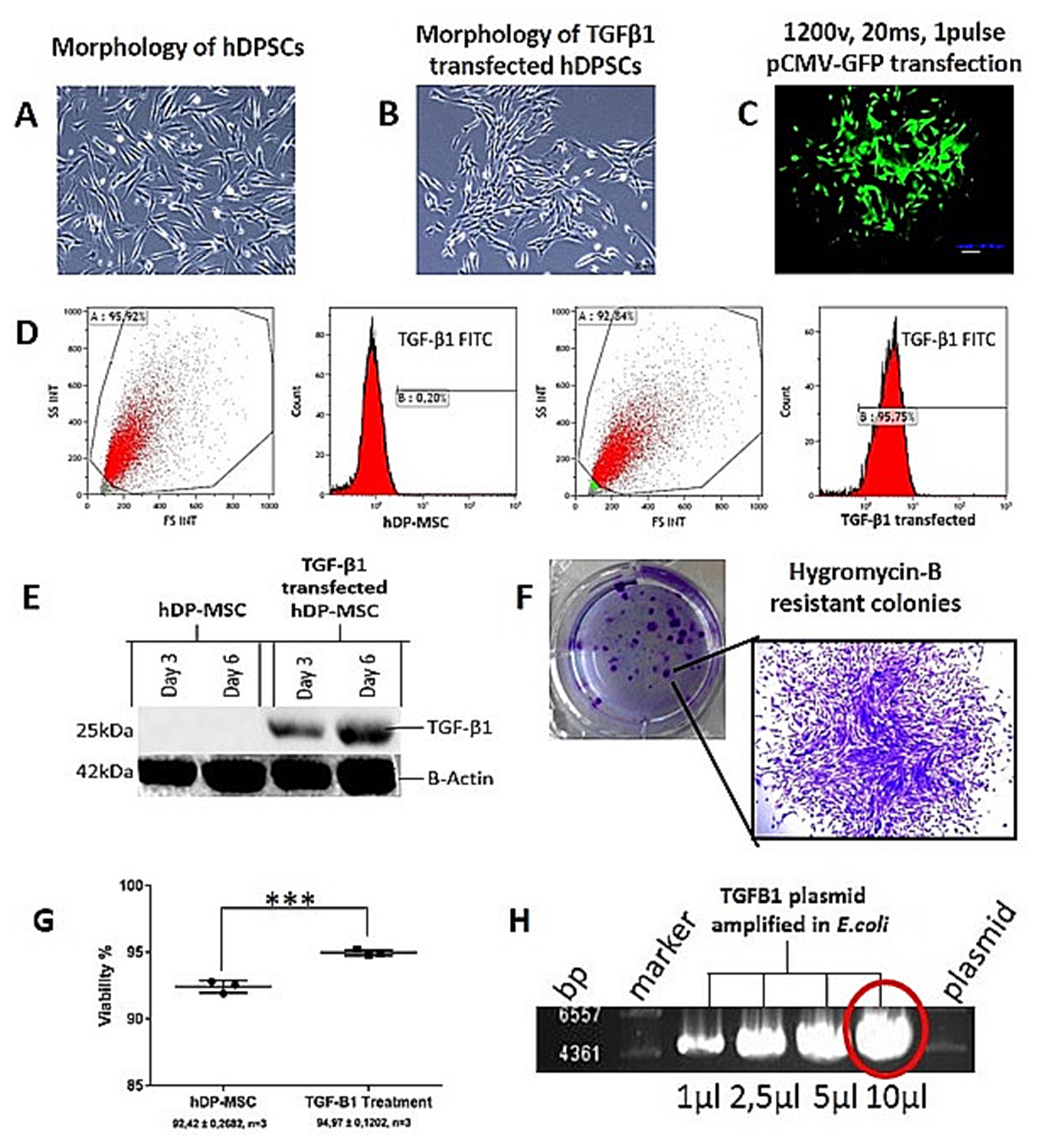
hDPSCs were isolated from human teeth, and were cultured with 20% Fetal Bovine Serum (FBS) in minimum essential media-alpha (α-MEM). TGF-β1 gene transfer into hDPSCs was performed by electroporation method after the plasmid was prepared. The transfection efficiency was achieved by using western blot and flow cytometry analyses and GFP transfection. Mesenchymal stem cell (MSC) markers, multilineage differentiation, cell proliferation, apoptosis, cell cycle, DNA damage and cellular senescence assays were performed by comparing the transfected and non-transfected cells. Statistical analyses were performed using GraphPad Prism.
RESULTS
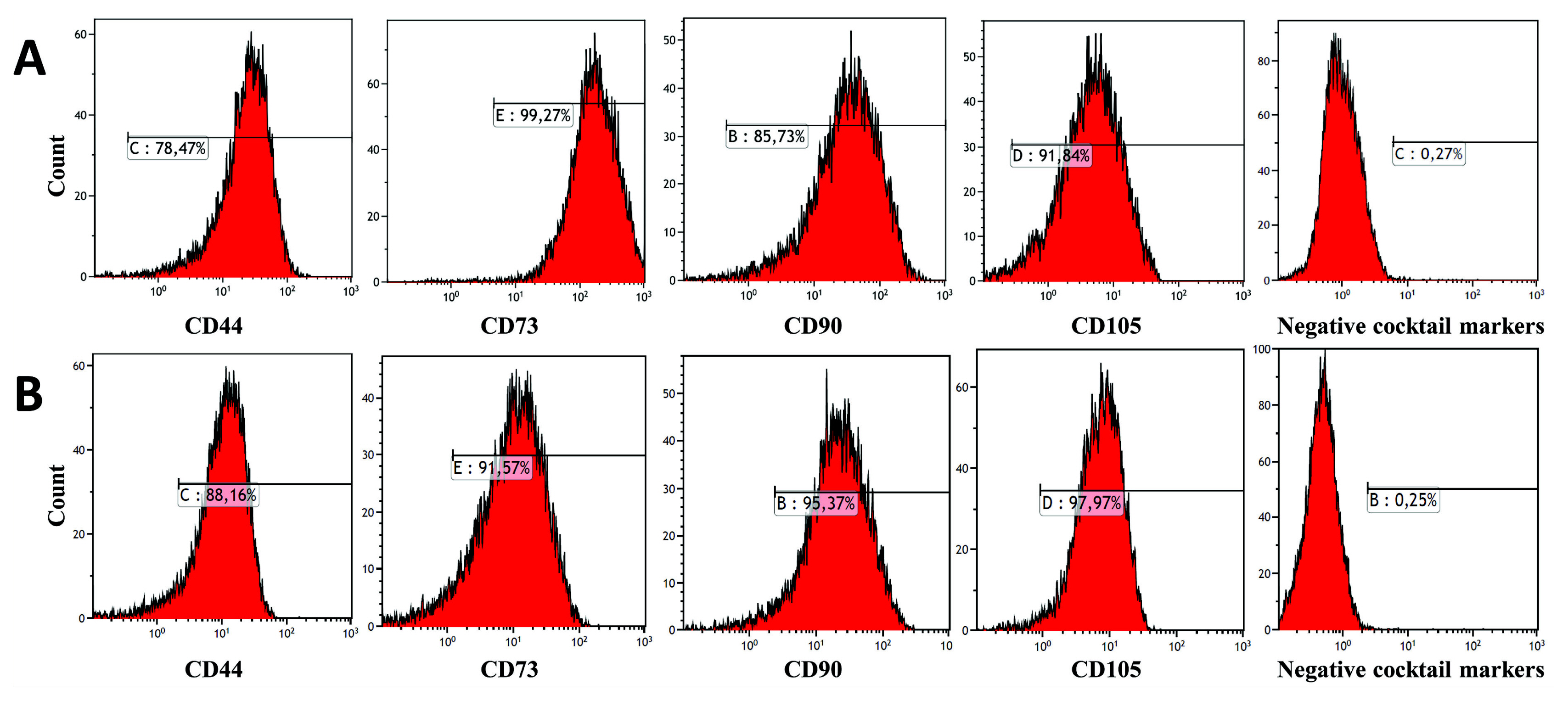
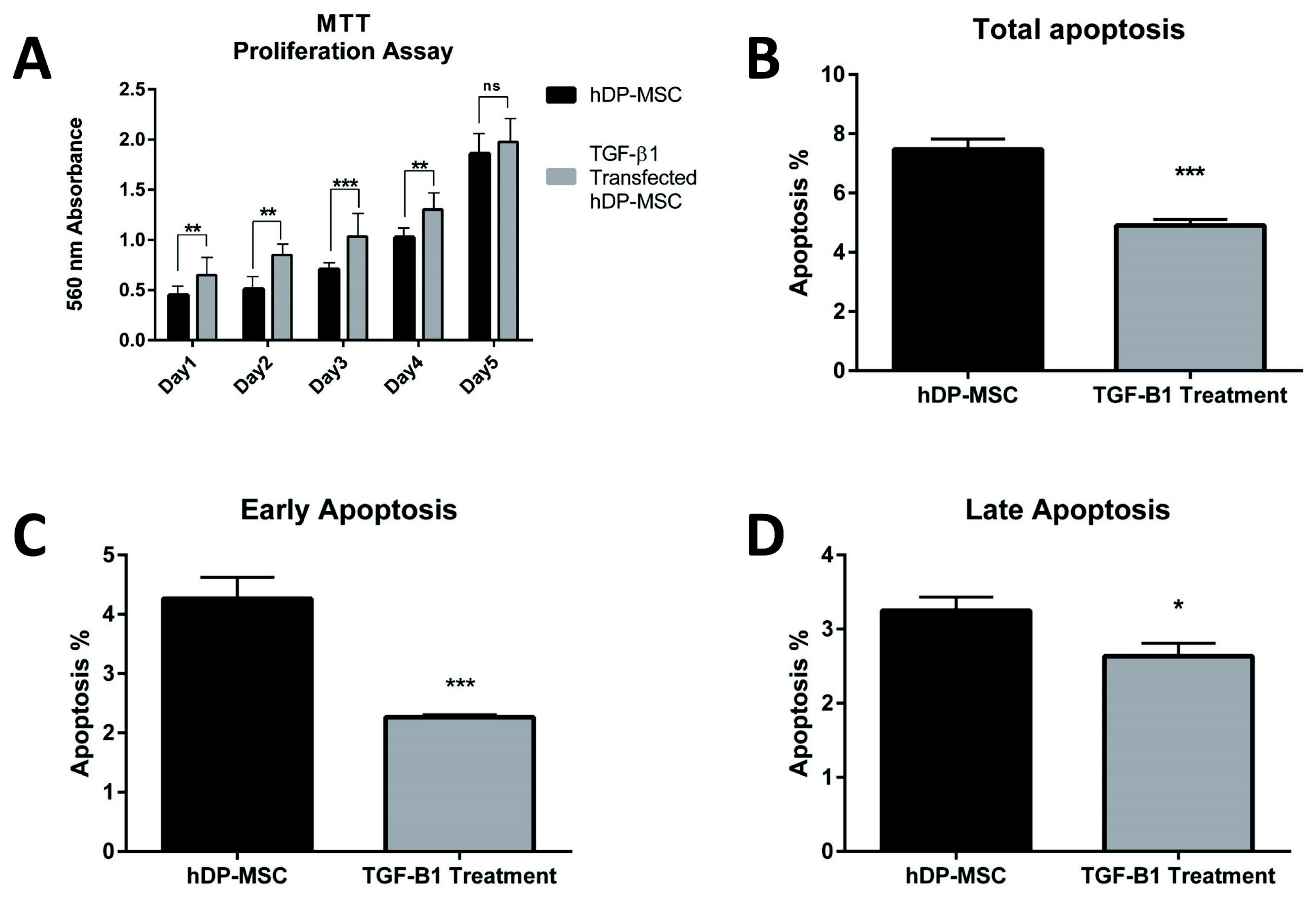
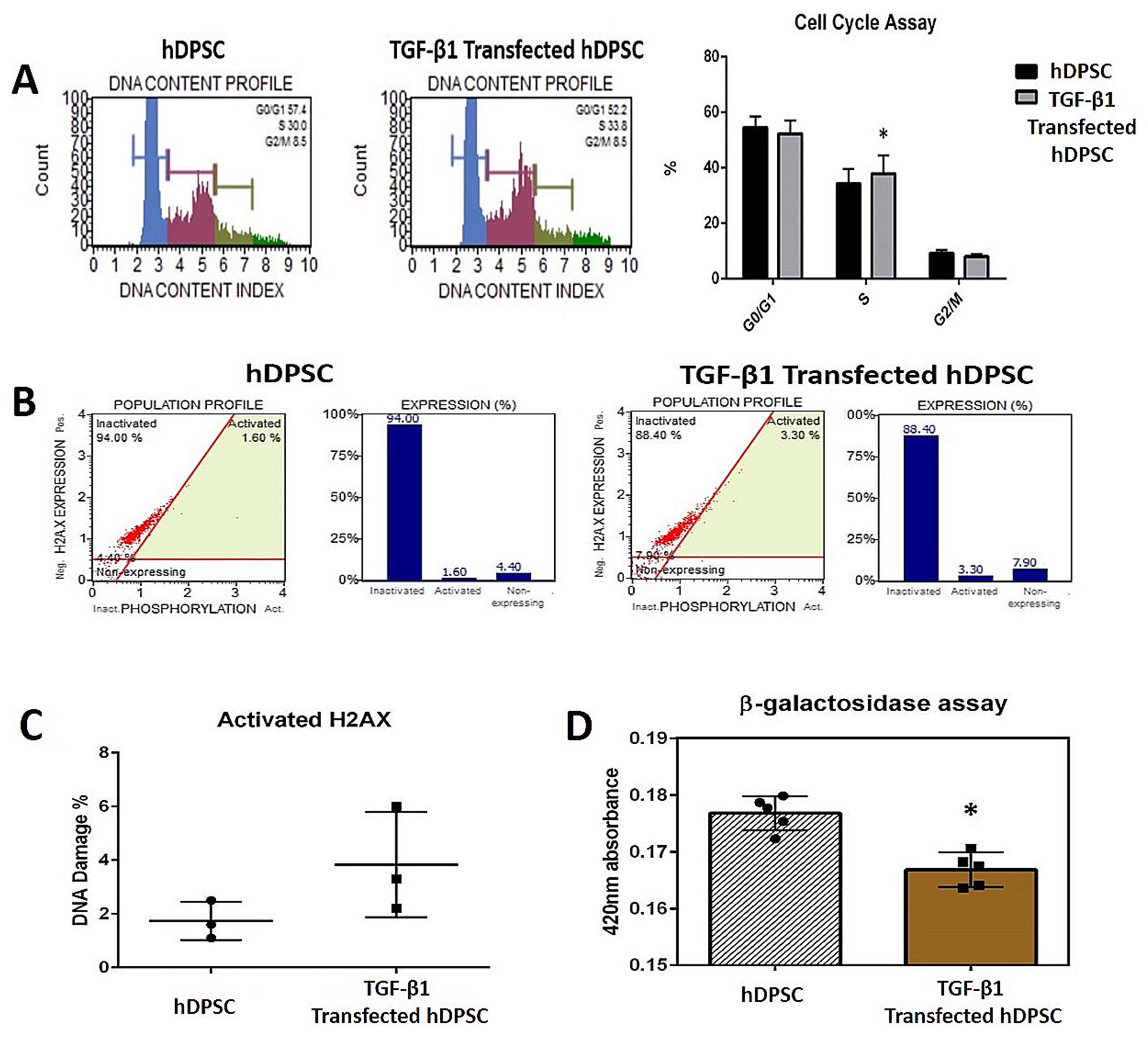
Strong expression of TGF-β1 in pCMV-TGF-β1-transfected hDPSCs was detected in flow cytometry analysis. TGF-β1 transfection efficiency was measured as 95%. Western blot analysis showed that TGF-β1 protein levels increased at third and sixth days in pCMV-TGF-β1-transfected hDPSCs. The continuous TGF-β1 overexpression in hDPSCs did not influence the immunophenotype and surface marker expression of MSCs. Our results showed that TGF-β1 increased osteogenic and chondrogenic differentiation, but decreased adipogenic differentiation. Overexpression of TGF-β1 increased the proliferation rate and decreased total apoptosis in hDPSCs (p<0.05). The number of cells at "S" phase was higher with TGF-β1 transfection (p<0.05). Cellular senescence decreased in TGF-β1 transfected group (p<0.05).
CONCLUSIONS
These results reflect that TGF-β1 has major impact on MSC differentiation. TGF-β1 transfection has positive effect on proliferation, cell cycle, and prevents cellular senescence and apoptosis.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Zhang F, Hong Y, Liang W, Ren T, Jing S, Lin J. Co-culture with Sertoli cells promotes proliferation and migration of umbilical cord mesenchymal stem cells. Biochem Biophys Res Commun. 2012; 427:86–90. DOI: 10.1016/j.bbrc.2012.09.007. PMID: 22975347.
Article2. Ferroni L, Gardin C, Tocco I, Epis R, Casadei A, Vindigni V, Mucci G, Zavan B. Potential for neural differentiation of mesenchymal stem cells. Adv Biochem Eng Biotechnol. 2013; 129:89–115.
Article3. Patel DM, Shah J, Srivastava AS. Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells Int. 2013; 2013; DOI: 10.1155/2013/496218. PMID: 23577036. PMCID: 3615627.
Article4. Karaöz E, Doğan BN, Aksoy A, Gacar G, Akyüz S, Ayhan S, Genç ZS, Yürüker S, Duruksu G, Demircan PC, Sariboyaci AE. Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem Cell Biol. 2010; 133:95–112. DOI: 10.1007/s00418-009-0646-5. PMID: 19816704.
Article5. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000; 97:13625–13630. DOI: 10.1073/pnas.240309797. PMID: 11087820. PMCID: 17626.6. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003; 100:5807–5812. DOI: 10.1073/pnas.0937635100. PMID: 12716973. PMCID: 156282.
Article7. d’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, Papaccio G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007; 14:1162–1171. DOI: 10.1038/sj.cdd.4402121. PMID: 17347663.
Article8. Alge DL, Zhou D, Adams LL, Wyss BK, Shadday MD, Woods EJ, Gabriel Chu TM, Goebel WS. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med. 2010; 4:73–81. DOI: 10.1002/term.220. PMID: 19842108. PMCID: 2830796.
Article9. Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009; 88:792–806. DOI: 10.1177/0022034509340867. PMID: 19767575. PMCID: 2830488.
Article10. Rizk A, Rabie BM. Electroporation for transfection and differentiation of dental pulp stem cells. Biores Open Access. 2013; 2:155–162. DOI: 10.1089/biores.2012.0273. PMID: 23593568. PMCID: 3620497.
Article11. Liang YY, Brunicardi FC, Lin X. Smad3 mediates immediate early induction of Id1 by TGF-beta. Cell Res. 2009; 19:140–148. DOI: 10.1038/cr.2008.321. PMID: 19079362.
Article12. Massagué J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000; 1:169–178. DOI: 10.1038/35043051. PMID: 11252892.13. Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005; 118:3573–3584. DOI: 10.1242/jcs.02554. PMID: 16105881.14. Siegel PM, Massagué J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003; 3:807–821. DOI: 10.1038/nrc1208. PMID: 14557817.
Article15. Frippiat C, Chen QM, Zdanov S, Magalhaes JP, Remacle J, Toussaint O. Subcytotoxic H2O2 stress triggers a release of transforming growth factor-beta 1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J Biol Chem. 2001; 276:2531–2537. DOI: 10.1074/jbc.M006809200. PMID: 11060295.
Article16. Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, Li S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials. 2011; 32:3921–3930. DOI: 10.1016/j.biomaterials.2011.02.019. PMID: 21397942. PMCID: 3073995.
Article17. Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, Choong C, Yang Z, Vemuri MC, Rao MS, Tanavde V. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008; 112:295–307. DOI: 10.1182/blood-2007-07-103697. PMID: 18332228.
Article18. Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000; 275:36803–36810. DOI: 10.1074/jbc.M005912200. PMID: 10969078.
Article19. Carr BI, Hayashi I, Branum EL, Moses HL. Inhibition of DNA synthesis in rat hepatocytes by platelet-derived type beta transforming growth factor. Cancer Res. 1986; 46:2330–2334. PMID: 3008986.20. Principe DR, Doll JA, Bauer J, Jung B, Munshi HG, Bartholin L, Pasche B, Lee C, Grippo PJ. TGF-β: duality of function between tumor prevention and carcinogenesis. J Natl Cancer Inst. 2014; 106:DOI: 10.1093/jnci/djt369. PMID: 24511106. PMCID: 3952197.
Article21. Burova E, Borodkina A, Shatrova A, Nikolsky N. Sublethal oxidative stress induces the premature senescence of human mesenchymal stem cells derived from endometrium. Oxid Med Cell Longev. 2013; 2013; DOI: 10.1155/2013/474931. PMID: 24062878. PMCID: 3767075.
Article22. Sarugaser R, Hanoun L, Keating A, Stanford WL, Davies JE. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS One. 2009; 4:DOI: 10.1371/journal.pone.0006498. PMID: 19652709. PMCID: 2714967.
Article23. Senturk S, Mumcuoglu M, Gursoy-Yuzugullu O, Cingoz B, Akcali KC, Ozturk M. Transforming growth factor-beta induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology. 2010; 52:966–974. DOI: 10.1002/hep.23769. PMID: 20583212.
Article24. Yu AL, Birke K, Moriniere J, Welge-Lüssen U. TGF-{beta}2 induces senescence-associated changes in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2010; 51:5718–5723. DOI: 10.1167/iovs.10-5679. PMID: 20554622.
Article25. Walenda G, Abnaof K, Joussen S, Meurer S, Smeets H, Rath B, Hoffmann K, Fröhlich H, Zenke M, Weiskirchen R, Wagner W. TGF-beta1 does not induce senescence of multipotent mesenchymal stromal cells and has similar effects in early and late passages. PLoS One. 2013; 8:DOI: 10.1371/journal.pone.0077656. PMID: 24147049. PMCID: 3798389.
Article26. Akpinar G, Kasap M, Aksoy A, Duruksu G, Gacar G, Karaoz E. Phenotypic and proteomic characteristics of human dental pulp derived mesenchymal stem cells from a natal, an exfoliated deciduous, and an impacted third molar tooth. Stem Cells Int. 2014; 2014; DOI: 10.1155/2014/457059. PMID: 25379041. PMCID: 4212660.
Article27. Jian H, Shen X, Liu I, Semenov M, He X, Wang XF. Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 2006; 20:666–674. DOI: 10.1101/gad.1388806. PMID: 16543220. PMCID: 1413283.
Article28. Ito T, Sawada R, Fujiwara Y, Seyama Y, Tsuchiya T. FGF-2 suppresses cellular senescence of human mesenchymal stem cells by down-regulation of TGF-beta2. Biochem Biophys Res Commun. 2007; 359:108–114. DOI: 10.1016/j.bbrc.2007.05.067. PMID: 17532297.
Article29. Debacq-Chainiaux F, Borlon C, Pascal T, Royer V, Eliaers F, Ninane N, Carrard G, Friguet B, de Longueville F, Boffe S, Remacle J, Toussaint O. Repeated exposure of human skin fibroblasts to UVB at subcytotoxic level triggers premature senescence through the TGF-beta1 signaling pathway. J Cell Sci. 2005; 118:743–758. DOI: 10.1242/jcs.01651. PMID: 15671065.
Article30. Wagner W, Ho AD. Mesenchymal stem cell preparations--comparing apples and oranges. Stem Cell Rev. 2007; 3:239–248. DOI: 10.1007/s12015-007-9001-1. PMID: 18074246.
Article31. Wu J, Niu J, Li X, Wang X, Guo Z, Zhang F. TGF-β1 induces senescence of bone marrow mesenchymal stem cells via increase of mitochondrial ROS production. BMC Dev Biol. 2014; 14:21. DOI: 10.1186/1471-213X-14-21. PMID: 24886313. PMCID: 4031602.
Article32. Kim YI, Ryu JS, Yeo JE, Choi YJ, Kim YS, Ko K, Koh YG. Overexpression of TGF-β1 enhances chondrogenic differentiation and proliferation of human synovium-derived stem cells. Biochem Biophys Res Commun. 2014; 450:1593–1599. DOI: 10.1016/j.bbrc.2014.07.045. PMID: 25035928.
Article33. Si X, Liu X, Li J, Wu X. Transforming growth factor-β1 promotes homing of bone marrow mesenchymal stem cells in renal ischemia-reperfusion injury. Int J Clin Exp Pathol. 2015; 8:12368–12378. PMID: 26722423. PMCID: 4680368.34. Liu Q, Cheng G, Wang Z, Zhan S, Xiong B, Zhao X. Bone marrow-derived mesenchymal stem cells differentiate into nerve-like cells in vitro after transfection with brain-derived neurotrophic factor gene. In Vitro Cell Dev Biol Anim. 2015; 51:319–327. DOI: 10.1007/s11626-015-9875-1. PMID: 25773996. PMCID: 4368845.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immune Tolerance of Human Dental Pulp-Derived Mesenchymal Stem Cells Mediated by CD4âºCD25âºFoxP3⺠Regulatory T-Cells and Induced by TGF-β1 and IL-10
- Characterization of Human Dental Pulp Cells from Supernumerary Teeth by Using Flow Cytometry Analysis
- Comparison of Transforming Growth Factor-Beta1 and Lovastatin on Differentiating Mesenchymal Stem Cells toward Nucleus Pulposus-like Phenotype: An In Vitro Cell Culture Study
- Morphological evaluation during in vitro chondrogenesis of dental pulp stromal cells
- Aspirin-Triggered Resolvin D1 Inhibits TGF-β1-Induced EndMT through Increasing the Expression of Smad7 and Is Closely Related to Oxidative Stress