Yonsei Med J.
2017 Sep;58(5):1031-1039. 10.3349/ymj.2017.58.5.1031.
Immune Tolerance of Human Dental Pulp-Derived Mesenchymal Stem Cells Mediated by CD4âºCD25âºFoxP3⺠Regulatory T-Cells and Induced by TGF-β1 and IL-10
- Affiliations
-
- 1Department of Plastic & Reconstructive Surgery, College of Medicine, Yonsei University, Seoul, Korea. hsaturn@hanmail.net
- 2Institute for Human Tissue Restoration, College of Medicine, Yonsei University, Seoul, Korea.
- 3Department of Orthodontics, College of Dentistry, Yonsei University, Seoul, Korea.
- 4Yujin Plastic Surgery, Seoul, Korea.
- KMID: 2418942
- DOI: http://doi.org/10.3349/ymj.2017.58.5.1031
Abstract
- PURPOSE
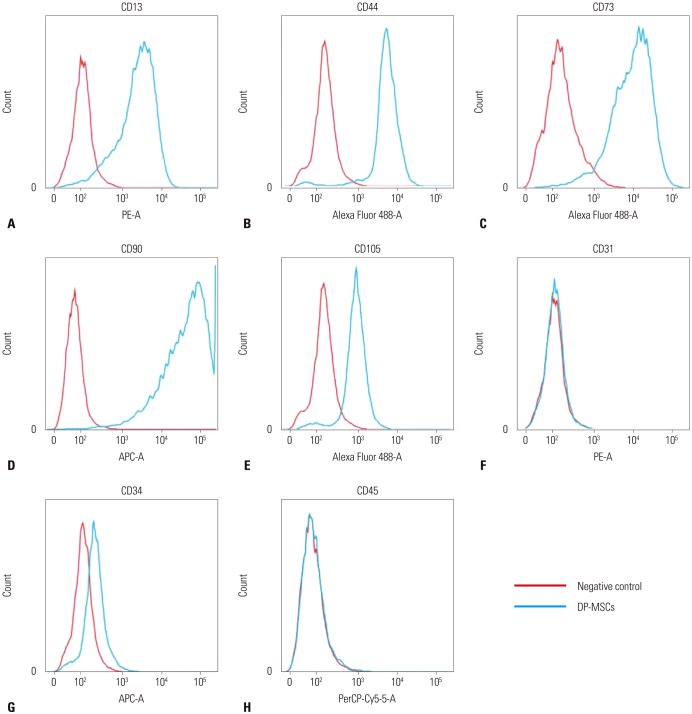
Most studies on immune tolerance of mesenchymal stem cells (MSCs) have been performed using MSCs derived from bone marrow, cord blood, or adipose tissue. MSCs also exist in the craniofacial area, specifically in teeth. The purpose of this study was to evaluate the mechanisms of immune tolerance of dental pulp-derived MSC (DP-MSC) in vitro and in vivo.
MATERIALS AND METHODS
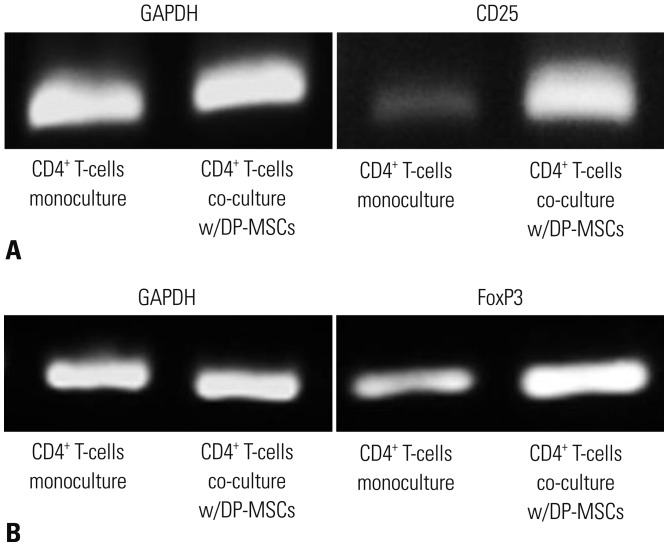
We isolated DP-MSCs from human dental pulp and co-cultured them with CD4⺠T-cells. To evaluate the role of cytokines, we blocked TGF-β and IL-10, separately and together, in co-cultured DP-MSCs and CD4⺠T-cells. We analyzed CD25 and FoxP3 to identify regulatory T-cells (Tregs) by fluorescence-activated cell sorting (FACS) and real-time PCR. We performed alloskin grafts with and without DP-MSC injection in mice. We performed mixed lymphocyte reactions (MLRs) to check immune tolerance.
RESULTS
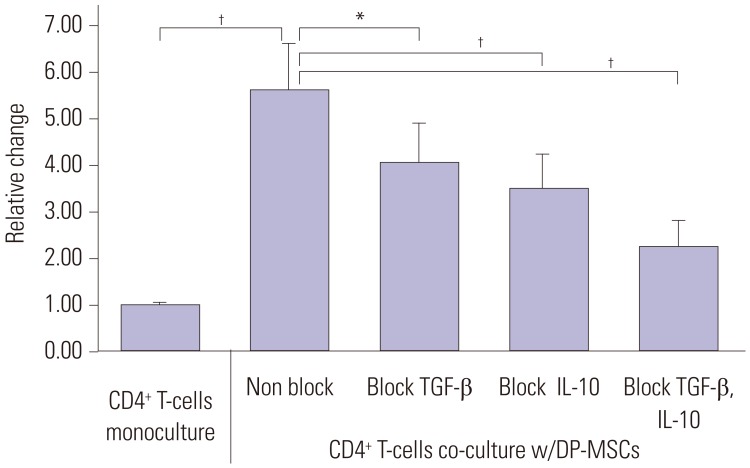
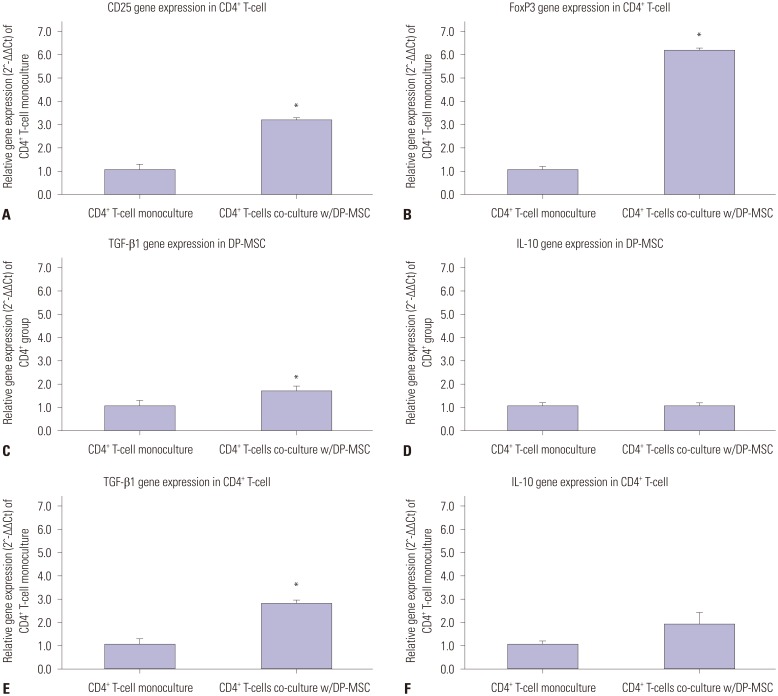
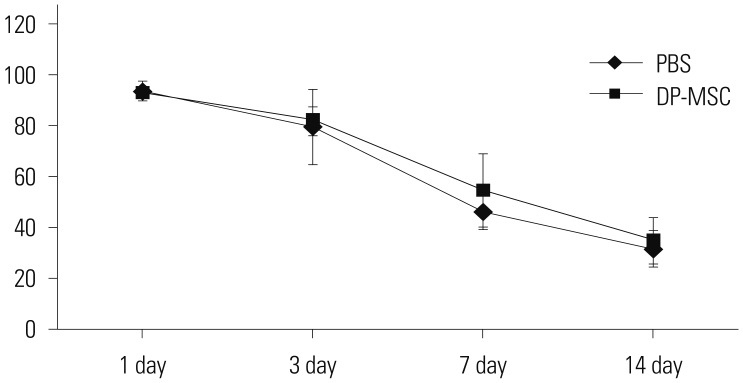
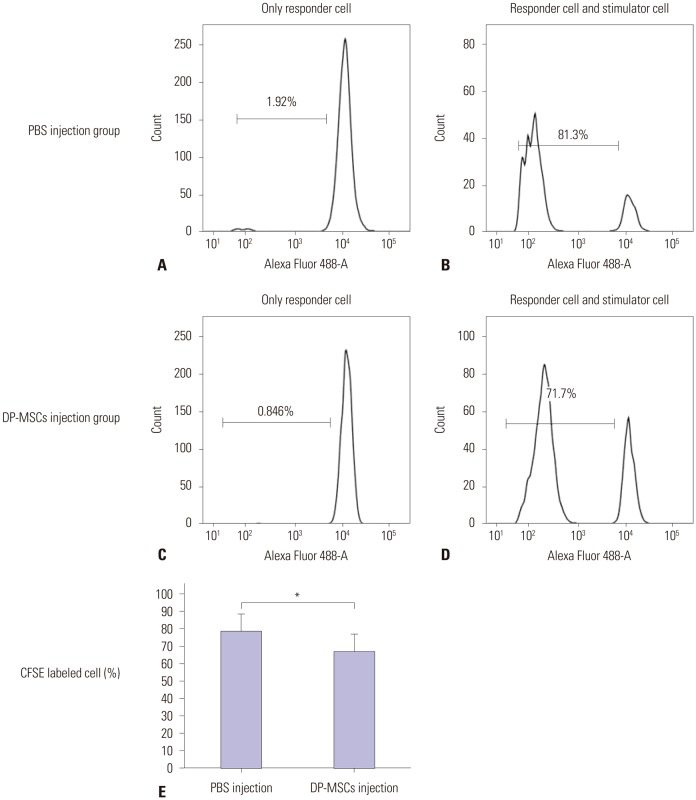
Co-culture of CD4⺠T-cells with DP-MSCs increased the number of CD4âºCD25âºFoxP3⺠Tregs (p<0.01). TGF-β or/and IL-10 blocking suppressed Treg induction in co-cultured cells (p<0.05). TGF-β1 mRNA levels were higher in co-cultured DP-MSCs and in co-cultured CD4⺠T-cells than in the respective monocultured cells. However, IL-10 mRNA levels were not different. There was no difference in alloskin graft survival rate and area between the DP-MSC injection group and the non-injection group. Nonetheless, MLR was reduced in the DP-MSC injected group (p<0.05).
CONCLUSION
DP-MSCs can modulate immune tolerance by increasing CD4âºCD25âºFoxP3⺠Tregs. TGF-β1 and IL-10 are factors in the immune-tolerance mechanism. Pure DP-MSC therapy may not be an effective treatment for rejection, although it may module immune tolerance in vivo.
Keyword
MeSH Terms
-
Allografts/immunology
Animals
Biomarkers/metabolism
CD4 Antigens/*metabolism
Cell Proliferation/drug effects
Coculture Techniques
Dental Pulp/*cytology
Forkhead Transcription Factors/metabolism
Graft Survival/immunology
Humans
*Immune Tolerance/drug effects
Interleukin-10/*pharmacology
Interleukin-2 Receptor alpha Subunit/*metabolism
Lymphocyte Culture Test, Mixed
Mesenchymal Stem Cell Transplantation
Mesenchymal Stromal Cells/cytology/*immunology
Mice, Inbred BALB C
Real-Time Polymerase Chain Reaction
Skin Transplantation
T-Lymphocytes, Regulatory/drug effects/*immunology
Transforming Growth Factor beta1/*pharmacology
Biomarkers
CD4 Antigens
Forkhead Transcription Factors
Interleukin-2 Receptor alpha Subunit
Transforming Growth Factor beta1
Interleukin-10
Figure
Reference
-
1. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000; 97:13625–13630. PMID: 11087820.2. Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002; 81:531–535. PMID: 12147742.
Article3. Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y, Gronthos S, et al. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther. 2010; 1:5. PMID: 20504286.
Article4. Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003; 5:485–489. PMID: 14660044.
Article5. Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008; 26:212–222. PMID: 17932417.
Article6. Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008; 36:309–318. PMID: 18279718.7. English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009; 156:149–160. PMID: 19210524.8. Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011; 164:1–8.
Article9. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006; 24:1294–1301. PMID: 16410387.
Article10. Tark KC, Hong JW, Kim YS, Hahn SB, Lee WJ, Lew DH. Effects of human cord blood mesenchymal stem cells on cutaneous wound healing in leprdb mice. Ann Plast Surg. 2010; 65:565–572. PMID: 20948411.11. Lequeux C, Rodriguez J, Boucher F, Rouyer O, Damour O, Mojallal A, et al. In vitro and in vivo biocompatibility, bioavailability and tolerance of an injectable vehicle for adipose-derived stem/stromal cells for plastic surgery indications. J Plast Reconstr Aesthet Surg. 2015; 68:1491–1497. PMID: 26282247.12. Khalifian S, Brazio PS, Mohan R, Shaffer C, Brandacher G, Barth RN, et al. Facial transplantation: the first 9 years. Lancet. 2014; 384:2153–2163. PMID: 24783986.
Article13. Sarhane KA, Tuffaha SH, Broyles JM, Ibrahim AE, Khalifian S, Baltodano P, et al. A critical analysis of rejection in vascularized composite allotransplantation: clinical, cellular and molecular aspects, current challenges, and novel concepts. Front Immunol. 2013; 4:406. PMID: 24324470.
Article14. Plock JA, Schnider JT, Zhang W, Schweizer R, Tsuji W, Kostereva N, et al. Adipose- and bone marrow-derived mesenchymal stem cells prolong graft survival in vascularized composite allotransplantation. Transplantation. 2015; 99:1765–1773. PMID: 26102613.
Article15. Ding G, Liu Y, An Y, Zhang C, Shi S, Wang W, et al. Suppression of T cell proliferation by root apical papilla stem cells in vitro. Cells Tissues Organs. 2010; 191:357–364. PMID: 20090301.
Article16. Wada N, Menicanin D, Shi S, Bartold PM, Gronthos S. Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol. 2009; 219:667–676. PMID: 19160415.
Article17. Zhao Y, Wang L, Jin Y, Shi S. Fas ligand regulates the immunomodulatory properties of dental pulp stem cells. J Dent Res. 2012; 91:948–954. PMID: 22904205.
Article18. Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation. 2005; 80:836–842. PMID: 16210973.
Article19. Yang H, Shin S, Ahn J, Choi Y, Kim KH, Chung CJ. Local injection of pulp cells enhances wound healing during the initial proliferative phase through the stimulation of host angiogenesis. J Endod. 2013; 39:788–794. PMID: 23683280.20. Huang GT, Sonoyama W, Chen J, Park SH. In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res. 2006; 324:225–236. PMID: 16440193.
Article21. Park SH, Hsiao GY, Huang GT. Role of substance P and calcitonin gene-related peptide in the regulation of interleukin-8 and monocyte chemotactic protein-1 expression in human dental pulp. Int Endod J. 2004; 37:185–192. PMID: 15009408.
Article22. Chung CR, Kim HN, Park Y, Kim MJ, Oh YJ, Shin SJ, et al. Morphological evaluation during in vitro chondrogenesis of dental pulp stromal cells. Restor Dent Endod. 2012; 37:34–40.23. Takeda T, Tezuka Y, Horiuchi M, Hosono K, Iida K, Hatakeyama D, et al. Characterization of dental pulp stem cells of human tooth germs. J Dent Res. 2008; 87:676–681. PMID: 18573990.
Article24. Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009; 88:792–806. PMID: 19767575.25. Ichim TE, Harman RJ, Min WP, Minev B, Solano F, Rodriguez JP, et al. Autologous stromal vascular fraction cells: a tool for facilitating tolerance in rheumatic disease. Cell Immunol. 2010; 264:7–17. PMID: 20537320.
Article26. Ma OK, Chan KH. Immunomodulation by mesenchymal stem cells: Interplay between mesenchymal stem cells and regulatory lymphocytes. World J Stem Cells. 2016; 8:268–278. PMID: 27679683.
Article27. Jeong SH, Ji YH, Yoon ES. Immunosuppressive activity of adipose tissue-derived mesenchymal stem cells in a rat model of hind limb allotransplantation. Transplant Proc. 2014; 46:1606–1614. PMID: 24935335.
Article28. Sloan AJ, Smith AJ. Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis. 2007; 13:151–157. PMID: 17305615.
Article29. Wang L, Zhao Y, Shi S. Interplay between mesenchymal stem cells and lymphocytes: implications for immunotherapy and tissue regeneration. J Dent Res. 2012; 91:1003–1010. PMID: 22988011.30. Valencic E, Loganes C, Cesana S, Piscianz E, Gaipa G, Biagi E, et al. Inhibition of mesenchymal stromal cells by pre-activated lymphocytes and their culture media. Stem Cell Res Ther. 2014; 5:3. PMID: 24405828.
Article31. Lee SM, Lee SC, Kim SJ. Contribution of human adipose tissue-derived stem cells and the secretome to the skin allograft survival in mice. J Surg Res. 2014; 188:280–289. PMID: 24560349.
Article32. Casiraghi F, Perico N, Remuzzi G. Mesenchymal stromal cells to promote solid organ transplantation tolerance. Curr Opin Organ Transplant. 2013; 18:51–58. PMID: 23254705.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- IL-17A-Producing Foxp3⺠Regulatory T Cells and Human Diseases
- Regulatory T Cells in Hepatitis B and C Virus Infections
- Effects of TGF-β1 Overexpression on Biological Characteristics of Human Dental Pulp-derived Mesenchymal Stromal Cells
- Combined Treatment with Methylprednisolone and Human Bone Marrow-Derived Mesenchymal Stem Cells Ameliorate Experimental Autoimmune Encephalomyelitis
- Characterization of Human Dental Pulp Cells from Supernumerary Teeth by Using Flow Cytometry Analysis