Yonsei Med J.
2019 Jun;60(6):509-516. 10.3349/ymj.2019.60.6.509.
Induction of Melanoma Cell-Selective Apoptosis Using Anti-HER2 Antibody-Conjugated Gold Nanoparticles
- Affiliations
-
- 1Department of Oral & Maxillofacial Surgery, School of Dentistry, Pusan National University, Yangsan, Korea. kuksjs@pusan.ac.kr
- 2Department of Oral Anatomy, School of Dentistry, Pusan National University, Yangsan, Korea. ki91000m@pusan.ac.kr
- 3Feagle Co., Ltd., Yangsan, Korea.
- KMID: 2446955
- DOI: http://doi.org/10.3349/ymj.2019.60.6.509
Abstract
- PURPOSE
This study was conducted to verify the induction and mechanism of selective apoptosis in G361 melanoma cells using anti-HER2 antibody-conjugated gold nanoparticles (GNP-HER2).
MATERIALS AND METHODS
Following GNP-HER2 treatment of G361 cells, cell cycle arrest and apoptosis were measured by WST-1 assay, Hemacolor staining, Hoechst staining, immunofluorescence staining, fluorescence-activated cell sorting analysis, and Western blotting.
RESULTS
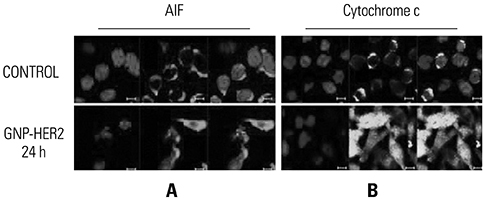
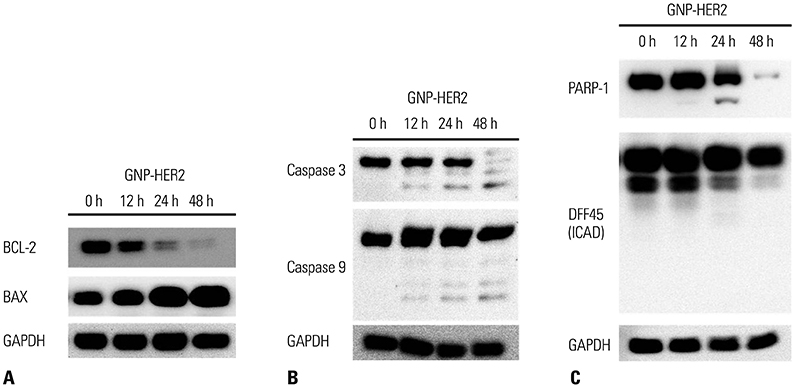
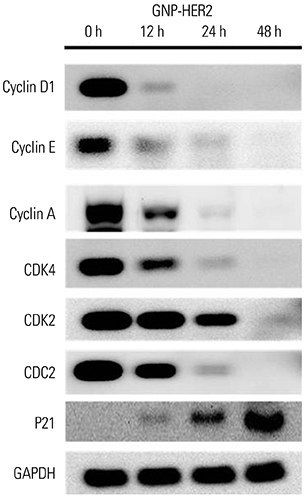
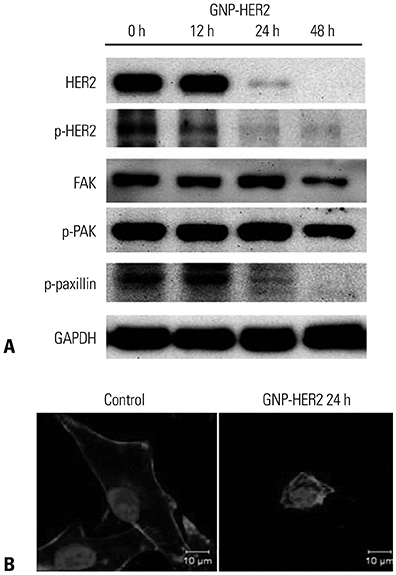
G361 cells treated with GNP-HER2 showed condensation of nuclei, which is an apoptotic phenomenon, and translocation of apoptosis-inducing factor and cytochrome c from mitochondria into the nucleus and cytoplasm, respectively. Increases in BAX in cells undergoing apoptosis, activation of caspase-3 and -9, and fragmentation of poly (ADP-ribose) polymerase and DNA fragmentation factor 45 (inhibitor of caspase-activated DNase) were observed upon GNP-HER2 treatment. Following GNP-HER2 treatment, an increase of cells in sub-G1 phase, which is a signal of cell apoptosis, was observed. This resulted in the down-regulation of cyclin A, cyclin D1, cyclin E, cdk2, cdk4, and cdc2 and the up-regulation of p21. Thus, GNP-HER2 treatment was confirmed to induce the cessation of cell cycle progression. Also, decreases in phospho-focal adhesion kinase and phospho-human epidermal growth factor receptor, which activate cellular focal adhesion, and decreases in phospho-paxillin, which stimulates the disassembly of filamentous actin, were observed. Reduced cell adhesion and disassembly of the intracellular structure indicated cell deactivation.
CONCLUSION
GNP-HER2 can selectively kill G361 melanoma cells without affecting normal cells. The mechanism of G361 cell death upon treatment with GNP-HER2 was apoptosis accompanied by activation of caspases.
Keyword
MeSH Terms
-
Actins
Apoptosis Inducing Factor
Apoptosis*
Blotting, Western
Caspase 3
Caspases
Cell Adhesion
Cell Cycle
Cell Cycle Checkpoints
Cell Death
Cyclin A
Cyclin D1
Cyclin E
Cyclins
Cytochromes c
Cytoplasm
DNA Fragmentation
Down-Regulation
Flow Cytometry
Fluorescent Antibody Technique
Focal Adhesions
Melanoma*
Mitochondria
Nanoparticles*
Phosphotransferases
Receptor, Epidermal Growth Factor
Up-Regulation
Actins
Apoptosis Inducing Factor
Caspase 3
Caspases
Cyclin A
Cyclin D1
Cyclin E
Cyclins
Cytochromes c
Phosphotransferases
Receptor, Epidermal Growth Factor
Figure
Reference
-
1. Kim SH, Hashimoto Y, Cho SN, Roszik J, Milton DR, Dal F, et al. Microsomal PGE2 synthase-1 regulates melanoma cell survival and associates with melanoma disease progression. Pigment Cell Melanoma Res. 2016; 29:297–308.
Article2. Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003; 22:3138–3151.
Article3. Service RF. Materials and biology. Nanotechnology takes aim at cancer. Science. 2005; 310:1132–1134.
Article4. Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004; 77:126–134.
Article5. Shi Kam NW, Jessop TC, Wender PA, Dai H. Nanotube molecular transporters: internalization of carbon nanotube-protein conjugates into Mammalian cells. J Am Chem Soc. 2004; 126:6850–6851.
Article6. Magrez A, Kasas S, Salicio V, Pasquier N, Seo JW, Celio M, et al. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006; 6:1121–1125.
Article7. Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004; 4:11–18.
Article8. Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol. 2003; 21:41–46.
Article9. Ahamed M, Akhtar MJ, Raja M, Ahmad I, Siddiqui MK, AlSalhi MS, et al. ZnO nanorod-induced apoptosis in human alveolar adenocarcinoma cells via p53, survivin and bax/bcl-2 pathways: role of oxidative stress. Nanomedicine. 2011; 7:904–913.
Article10. Arya G, Vandana M, Acharya S, Sahoo SK. Enhanced antiproliferative activity of Herceptin (HER2)-conjugated gemcitabine-loaded chitosan nanoparticle in pancreatic cancer therapy. Nanomedicine. 2011; 7:859–870.
Article11. Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005; 1:325–327.
Article12. Thomas M, Klibanov AM. Conjugation to gold nanoparticles enhances polyethylenimine's transfer of plasmid DNA into mammalian cells. Proc Natl Acad Sci U S A. 2003; 100:9138–9143.
Article13. Rosenberg SJ, Loening SA, Hawtrey CE, Narayana AS, Culp DA. Radical prostatectomy with adjuvant radioactive gold for prostatic cancer: a preliminary report. J Urol. 1985; 133:225–227.
Article14. Patra HK, Banerjee S, Chaudhuri U, Lahiri P, Dasgupta AK. Cell selective response to gold nanoparticles. Nanomedicine. 2007; 3:111–119.
Article15. El-Sayed IH, Huang X, El-Sayed MA. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 2005; 5:829–834.
Article16. El-Sayed IH, Huang X, El-Sayed MA. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006; 239:129–135.
Article17. Bargmann CI, Hung MC, Weinberg RA. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986; 319:226–230.
Article18. Rubin I, Yarden Y. The basic biology of HER2. Ann Oncol. 2001; 12:Suppl 1. S3–S8.
Article19. Neve RM, Lane HA, Hynes NE. The role of overexpressed HER2 in transformation. Ann Oncol. 2001; 12:Suppl 1. S9–S13.
Article20. Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990; 61:203–212.
Article21. Kämmerer U, Thanner F, Kapp M, Dietl J, Sütterlin M. Expression of tumor markers on breast and ovarian cancer cell lines. Anticancer Res. 2003; 23:1051–1055.22. Nakamura H, Saji H, Ogata A, Hosaka M, Hagiwara M, Kawasaki N, et al. Correlation between encoded protein overexpression and copy number of the HER2 gene with survival in non-small cell lung cancer. Int J Cancer. 2003; 103:61–66.
Article23. Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest. 2001; 19:554–568.24. Oehler MK, Brand A, Wain GV. Molecular genetics and endometrial cancer. J Br Menopause Soc. 2003; 9:27–31.
Article25. Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997; 88:347–354.
Article26. Nagata S. Apoptosis by death factor. Cell. 1997; 88:355–365.
Article27. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Apoptosis: programmed cell death eliminates unwanted cells. Molecular biology of the cell. 5th ed. New York: Garland Science;2008.28. Sabour Alaoui S, Dessirier V, de Araujo E, Alexaki VI, Pelekanou V, Lkhider M, et al. TWEAK affects keratinocyte G2/M growth arrest and induces apoptosis through the translocation of the AIF protein to the nucleus. PLoS One. 2012; 7:e33609.
Article29. Arias-González I, García-Carrancà AM, Cornejo-Garrido J, Ordaz-Pichardo C. Cytotoxic effect of Kalanchoe flammea and induction of intrinsic mitochondrial apoptotic signaling in prostate cancer cells. J Ethnopharmacol. 2018; 222:133–147.
Article30. Fan Y, Chiu JF, Liu J, Deng Y, Xu C, Zhang J, et al. Resveratrol induces autophagy-dependent apoptosis in HL-60 cells. BMC Cancer. 2018; 18:581.
Article31. Luo T, Yuan Y, Yu Q, Liu G, Long M, Zhang K, et al. PARP-1 overexpression contributes to Cadmium-induced death in rat proximal tubular cells via parthanatos and the MAPK signalling pathway. Sci Rep. 2017; 7:4331.
Article32. Errami Y, Brim H, Oumouna-Benachour K, Oumouna M, Naura AS, Kim H, et al. ICAD deficiency in human colon cancer and predisposition to colon tumorigenesis: linkage to apoptosis resistance and genomic instability. PLoS One. 2013; 8:e57871.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Study of EGFR Expression on Malignant Skin Tumor and Molecular Labelling Using Gold Nanoparticles
- Induction of Selective Cell Death of Oral Squamous Carcinoma Cells by Integrin alpha2 Antibody and EGFR Antibody
- Clinical Application of Gold Nanoparticles for Diagnosis and Treatment
- Trastuzumab-Conjugated Liposome-Coated Fluorescent Magnetic Nanoparticles to Target Breast Cancer
- Immunohistochemical Study of Malignant Melanoma with HMB - 45 Monoclonal Antibody and Anti S - 100 Protein Antibody