World J Mens Health.
2019 May;37(2):186-198. 10.5534/wjmh.180041.
C-Type Natriuretic Peptide/Natriuretic Peptide Receptor 2 Is Involved in Cell Proliferation and Testosterone Production in Mouse Leydig Cells
- Affiliations
-
- 1College of Basic Medical Science, Jiujiang, China. yangleigeili@163.com, 517159865@qq.com
- 2Clinical Skills Center, Affiliated Hospital of Jiujiang University, Jiujiang, China.
- 3Key Laboratory of System Bio-medicine of Jiangxi Province, Jiujiang University, Jiujiang, China.
- KMID: 2443234
- DOI: http://doi.org/10.5534/wjmh.180041
Abstract
- PURPOSE
This study investigated the role of natriuretic peptide receptor 2 (NPR2) on cell proliferation and testosterone secretion in mouse Leydig cells.
MATERIALS AND METHODS
Mouse testis of different postnatal stages was isolated to detect the expression C-type natriuretic peptide (CNP) and its receptor NPR2 by quantitative reverse transcription polymerase chain reaction (RT-qPCR). Leydig cells isolated from mouse testis were cultured and treated with shNPR2 lentiviruses or CNP. And then the cyclic guanosine monophosphate production, testosterone secretion, cell proliferation, cell cycle and cell apoptosis in mouse Leydig cells were analyzed by ELISA, RT-qPCR, Cell Counting Kit-8, and flow cytometry. Moreover, the expression of NPR2, cell cycle, apoptosis proliferation and cell cycle related gene were detected by RT-qPCR and Western blot.
RESULTS
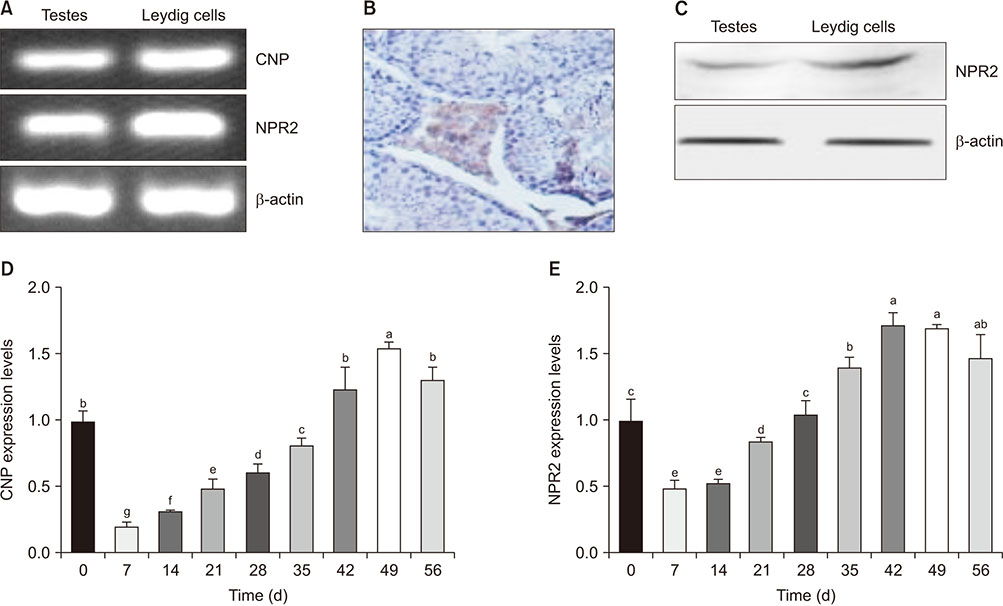
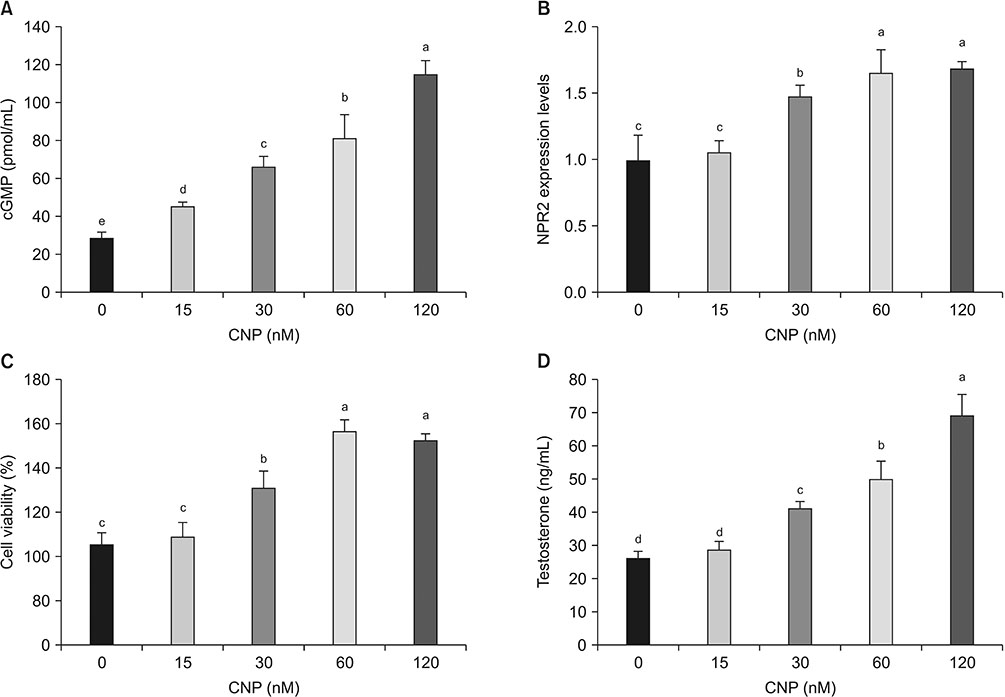
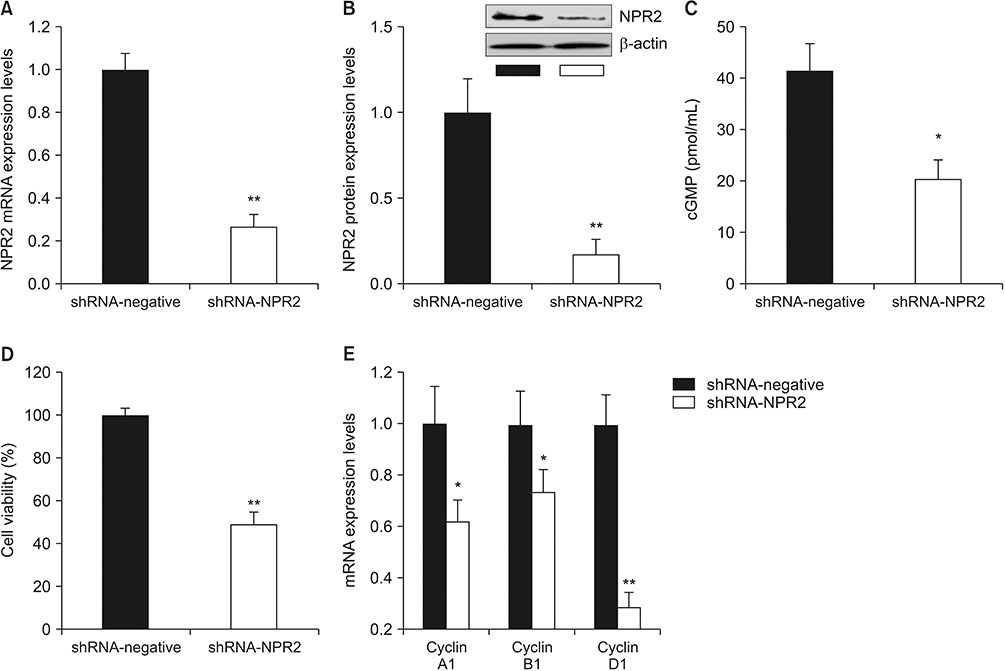
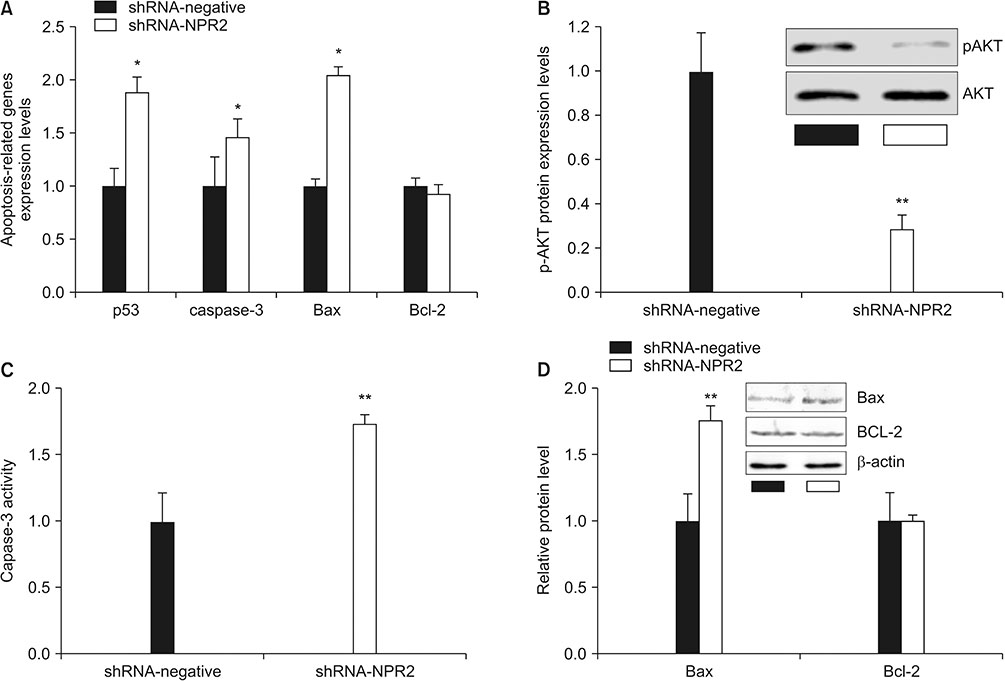
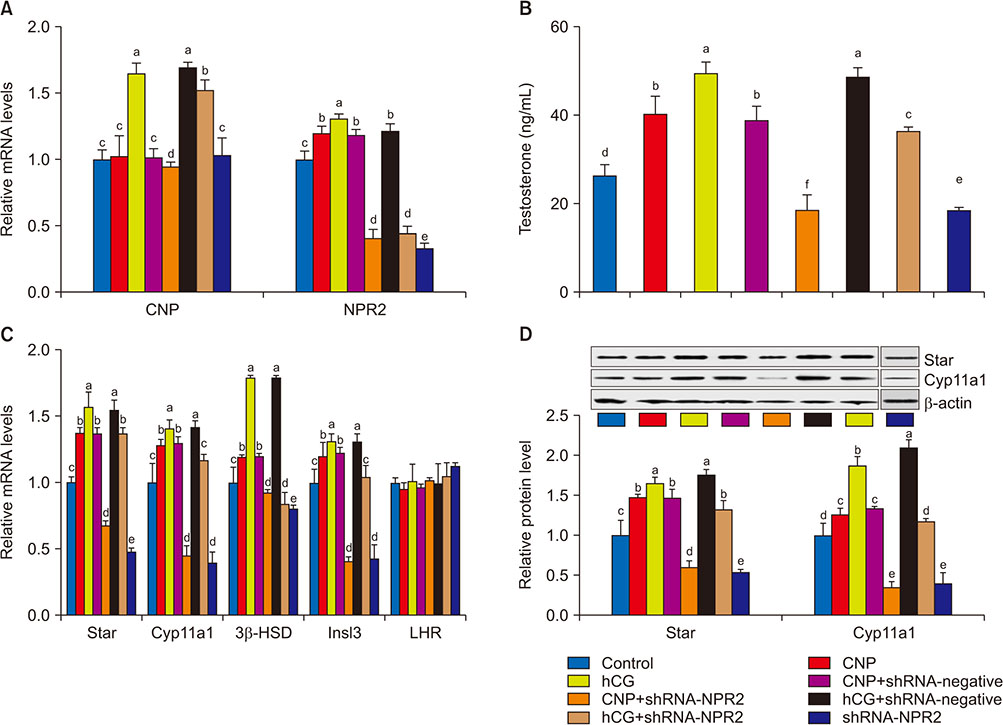
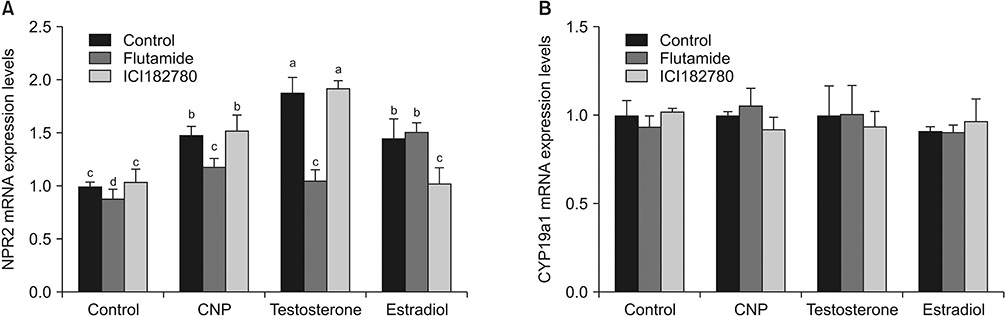
Knockdown of NPR2 by RNAi resulted in S phase cell cycle arrest, cell apoptosis, and decreased testosterone secretion in mouse Leydig cells.
CONCLUSIONS
Our study provides more evidences to better understand the function of CNP/NPR2 pathway in male reproduction, which may help us to treat male infertility.
Keyword
MeSH Terms
-
Animals
Apoptosis
Blotting, Western
Cell Count
Cell Cycle
Cell Cycle Checkpoints
Cell Proliferation*
Enzyme-Linked Immunosorbent Assay
Flow Cytometry
Germ Cells
Guanosine Monophosphate
Humans
Infertility, Male
Lentivirus
Leydig Cells*
Male
Mice*
Natriuretic Peptide, C-Type
Polymerase Chain Reaction
Receptors, Peptide*
Reproduction
Reverse Transcription
RNA Interference
S Phase
Testicular Diseases
Testis
Testosterone*
Guanosine Monophosphate
Natriuretic Peptide, C-Type
Receptors, Peptide
Testosterone
Figure
Reference
-
1. Ito A, Shirakawa H, Takumi N, Minegishi Y, Ohashi A, Howlader ZH, et al. Menaquinone-4 enhances testosterone production in rats and testis-derived tumor cells. Lipids Health Dis. 2011; 10:158.
Article2. Huhtaniemi IT. LH and FSH receptor mutations and their effects on puberty. Horm Res. 2002; 57:Suppl 2. 35–38.
Article3. Ferlin A, Bogatcheva NV, Gianesello L, Pepe A, Vinanzi C, Agoulnik AI, et al. Insulin-like factor 3 gene mutations in testicular dysgenesis syndrome: clinical and functional characterization. Mol Hum Reprod. 2006; 12:401–406.
Article4. Tremblay JJ. Molecular regulation of steroidogenesis in endocrine Leydig cells. Steroids. 2015; 103:3–10.
Article5. Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010; 330:366–369.
Article6. Yang L, Wei Q, Li W, Ge J, Zhao X, Ma B. C-type natriuretic peptide improved vitrified-warmed mouse cumulus oocyte complexes developmental competence. Cryobiology. 2016; 72:161–164.
Article7. Huang DH, Zhang SW, Zhao H, Zhang L. The role of C-type natriuretic peptide in rat testes during spermatogenesis. Asian J Androl. 2011; 13:275–280.
Article8. Sogawa C, Fujiwara Y, Tsukamoto S, Ishida Y, Yoshii Y, Furukawa T, et al. Mutant phenotype analysis suggests potential roles for C-type natriuretic peptide receptor (NPR-B) in male mouse fertility. Reprod Biol Endocrin. 2014; 12:64.
Article9. Kong N, Xu X, Zhang Y, Wang Y, Hao X, Zhao Y, et al. Natriuretic peptide type C induces sperm attraction for fertilization in mouse. Sci Rep. 2017; 7:39711.
Article10. Xia H, Chen Y, Wu KJ, Zhao H, Xiong CL, Huang DH. Role of C-type natriuretic peptide in the function of normal human sperm. Asian J Androl. 2016; 18:80–84.
Article11. Yang L, Wei Q, Li W, Xi Q, Zhao X, Ma B. NPR2 is involved in FSH-mediated mouse oocyte meiotic resumption. J Ovarian Res. 2016; 9:6.
Article12. Chen H, Gao L, Xiong Y, Yang D, Li C, Wang A, et al. Circadian clock and steroidogenic-related gene expression profiles in mouse Leydig cells following dexamethasone stimulation. Biochem Biophys Res Commun. 2017; 483:294–300.
Article13. Xia W, Mruk DD, Cheng CY. C-type natriuretic peptide regulates blood-testis barrier dynamics in adult rat testes. Proc Natl Acad Sci USA. 2007; 104:3841–3846.
Article14. Collin O, Lissbrant E, Bergh A. Atrial natriuretic peptide, brain natriuretic peptide and c-type natriuretic peptide: effects on testicular microcirculation and immunohistochemical localization. Int J Androl. 1997; 20:55–60.
Article15. Wang X, Wang H, Liu W, Zhang Z, Zhang Y, Zhang W, et al. High level of C-type natriuretic peptide induced by hyperandrogen-mediated anovulation in polycystic ovary syndrome mice. Clin Sci (Lond). 2018; 132:759–776.
Article16. Tsuji T, Kunieda T. A loss-of-function mutation in natriuretic peptide receptor 2 (Npr2) gene is responsible for disproportionate dwarfism in cn/cn mouse. J Biol Chem. 2005; 280:14288–14292.
Article17. Mericq V, Uyeda JA, Barnes KM, De LF, Baron J. Regulation of fetal rat bone growth by C-type natriuretic peptide and cGMP. Pediatr Res. 2000; 47:189–193.
Article18. Karan S, Frederick JW, Baehr W. Novel functions of photoreceptor guanylate cyclases revealed by targeted deletion. Mol Cell Biochem. 2010; 334:141–155.
Article19. Porter JG, Catalano R, Mcenroe G, Lewicki JA, Protter AA. C-type natriuretic peptide inhibits growth factor-dependent DNA synthesis in smooth muscle cells. Am J Physiol. 1992; 263:C1001–C1006.
Article20. Furuya M, Yoshida M, Hayashi Y, Ohnuma N, Minamino N, Kangawa K, et al. C-type natriuretic peptide is a growth inhibitor of rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1991; 177:927–931.
Article21. Khurana ML, Pandey KN. Receptor-mediated stimulatory effect of atrial natriuretic factor, brain natriuretic peptide, and C-type natriuretic peptide on testosterone production in purified mouse Leydig cells: activation of cholesterol side-chain cleavage enzyme. Endocrinology. 1993; 133:2141–2149.
Article22. Fouchécourt S, Livera G, Messiaen S, Fumel B, Parent AS, Marine JC, et al. Apoptosis of sertoli cells after conditional ablation of murine double minute 2 (Mdm2) gene is p53-dependent and results in male sterility. Cell Death Differ. 2016; 23:521–530.
Article23. Zhao F, Wang N, Yi Y, Lin P, Tang K, Wang A, et al. Knockdown of CREB3/Luman by shRNA in mouse granulosa cells results in decreased estradiol and progesterone synthesis and promotes cell proliferation. Plos One. 2016; 11:e0168246.
Article24. Sifer C, Bénifla JL, Bringuier AF, Porcher R, Blanc-Layrac G, Madélénat P, et al. Could induced apoptosis of human granulosa cells predict in vitro fertilization-embryo transfer outcome? A preliminary study of 25 women. Eur J Obstet Gynecol Reprod Biol. 2002; 103:150–153.25. Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of bad couples survival signals to the cell-intrinsic death machinery. Cell. 1997; 91:231–241.
Article26. Hatano M, Migita T, Ohishi T, Shima Y, Ogawa Y, Morohashi KI, et al. SF-1 deficiency causes lipid accumulation in Leydig cells via suppression of STAR and CYP11A1. Endocrine. 2016; 54:484–496.
Article27. Moon HW, Park JW, Lee KW, Jeong HC, Choi JB, Choi SW, et al. Administration of Goji (lycium Chinense mill.) Extracts improves erectile function in old aged rat model. World J Mens Health. 2016; 35:43–50.
Article28. Zhang M, Su YQ, Sugiura K, Wigglesworth K, Xia G, Eppig JJ. Estradiol promotes and maintains cumulus cell expression of natriuretic peptide receptor 2 (npr2) and meiotic arrest in mouse oocytes in vitro. Endocrinology. 2011; 152:4377–4385.
Article29. Abney TO. The potential roles of estrogens in regulating Leydig cell development and function: a review. Steroids. 1999; 64:610–617.
Article30. Zhang J, Wei Q, Cai J, Zhao X, Ma B. Effect of C-type natriuretic peptide on maturation and developmental competence of goat oocytes matured in vitro. Plos One. 2015; 10:e0132318.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of atrial natriuretic peptide on the proliferation and activity of osteoblastic cells
- Biomarkers in Heart Failure: Focus on B-type Natriuretic Peptide
- Clinical Implication of B-type Natriuretic Peptide in the Elderly
- B-type natriuretic peptide may have a role in the management of patent ductus arteriosus
- B-type natriuretic peptide in anesthesia practice to predict adverse cardiovascular outcomes