Lab Anim Res.
2017 Dec;33(4):283-290. 10.5625/lar.2017.33.4.283.
Dendropanax morbifera Léveille extract ameliorates D-galactose-induced memory deficits by decreasing inflammatory responses in the hippocampus
- Affiliations
-
- 1Department of Anatomy, College of Veterinary Medicine and Institute of Veterinary Science, Kangwon National University, Chuncheon 24341, South Korea. jhchoi@kangwon.ac.kr
- 2Department of Anatomy and Cell Biology, College of Veterinary Medicine, and Research Institute for Veterinary Science, Seoul National University, Seoul 08826, South Korea.
- 3Department of Biochemistry and Molecular Biology, Research Institute of Oral Sciences, College of Dentistry, Gangneung-Wonju National University, Gangneung 25457, South Korea.
- KMID: 2402264
- DOI: http://doi.org/10.5625/lar.2017.33.4.283
Abstract
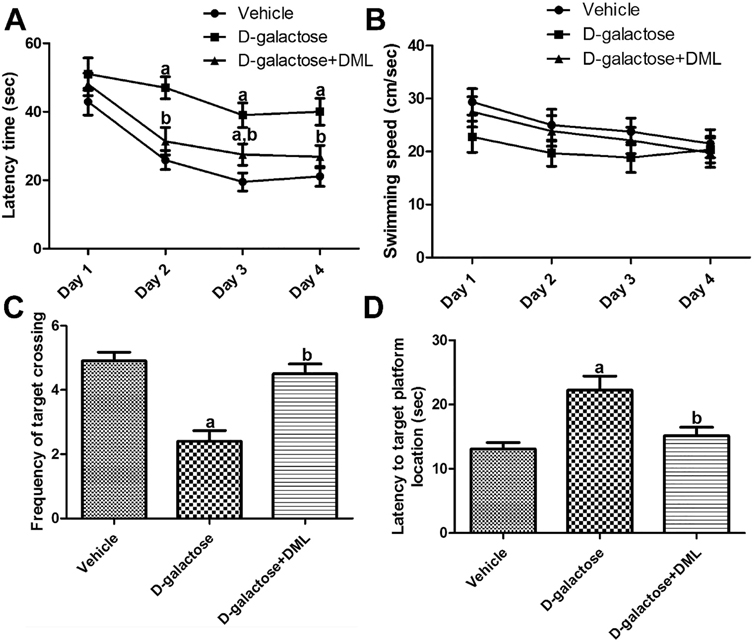
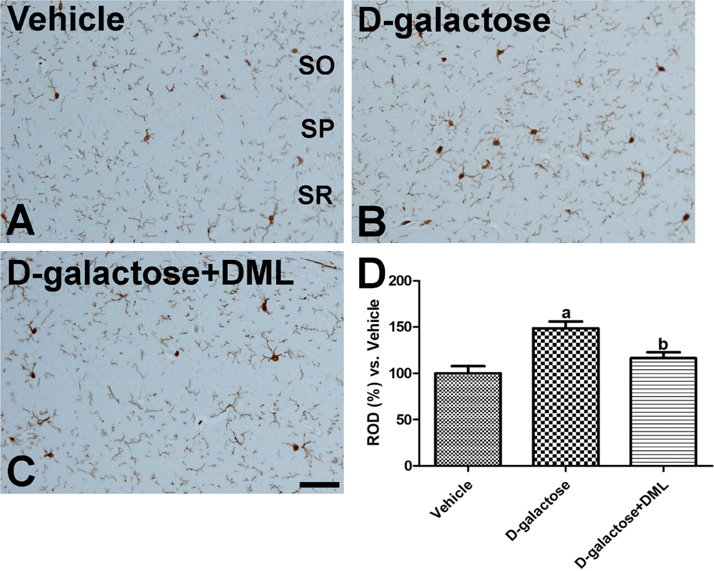
- In the present study, we examined the effects of Dendropanax morbifera Léveille leaf extract (DML) on D-galactose-induced morphological changes in microglia and cytokines, including pro-inflammatory cytokines (interleukin [IL]-1β, IL-6, and tumor necrosis factor [TNF]-α) and anti-inflammatory cytokines (IL-4 and IL-10) in the hippocampus. Administration of DML to D-galactose-treated mice significantly improved D-galactose-induced reduction in escape latency, swimming speed, and spatial preference for the target quadrant. In addition, administration of DML to D-galactose-treated mice significantly ameliorated the microglial activation and increases of IL-1β, IL-6, and TNF-α levels in the hippocampus. Administration of D-galactose significantly reduced IL-4 levels in the hippocampus, while administration of DML to D-galactose-treated mice significantly increased IL-4 level. However, we did not observe any significant changes in IL-10 levels in hippocampal homogenates. These results suggest that DML reduces D-galactose-induced mouse senescence by reducing pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α, as well as increasing anti-inflammatory cytokine IL-4.
MeSH Terms
Figure
Reference
-
1. Golden TR, Hinerfeld DA, Melov S. Oxidative stress and aging: beyond correlation. Aging Cell. 2002; 1(2):117–123.2. Joseph JA, Shukitt-Hale B, Casadesus G. Reversing the deleterious effects of aging on neuronal communication and behavior: beneficial properties of fruit polyphenolic compounds. Am J Clin Nutr. 2005; 81:1 Suppl. 313S–316S.3. Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009; 29(43):13435–13444.4. Kuzumaki N, Ikegami D, Imai S, Narita M, Tamura R, Yajima M, Suzuki A, Miyashita K, Niikura K, Takeshima H, Ando T, Ushijima T, Suzuki T, Narita M. Enhanced IL-1beta production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice. Synapse. 2010; 64(9):721–728.5. Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000; 51:245–270.6. Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhøj P, Pedersen BK. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999; 54(7):M357–M364.7. Norden DM, Godbout JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol. 2013; 39(1):19–34.8. Lu J, Zheng YL, Wu DM, Luo L, Sun DX, Shan Q. Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by D-galactose. Biochem Pharmacol. 2007; 74(7):1078–1090.9. Kim W, Kim DW, Yoo DY, Jung HY, Nam SM, Kim JW, Hong SM, Kim DW, Choi JH, Moon SM, Yoon YS, Hwang IK. Dendropanax morbifera Léveille extract facilitates cadmium excretion and prevents oxidative damage in the hippocampus by increasing antioxidant levels in cadmium-exposed rats. BMC Complement Altern Med. 2014; 14:428.10. Kim W, Kim DW, Yoo DY, Jung HY, Kim JW, Kim DW, Choi JH, Moon SM, Yoon YS, Hwang IK. Antioxidant effects of Dendropanax morbifera Léveille extract in the hippocampus of mercury-exposed rats. BMC Complement Altern Med. 2015; 15:247.11. Kim ES, Lee JS, Akram M, Kim KA, Shin YJ, Yu JH, Bae ON. Protective activity of Dendropanax morbifera against cisplatin-induced acute kidney injury. Kidney Blood Press Res. 2015; 40(1):1–12.12. Akram M, Kim KA, Kim ES, Syed AS, Kim CY, Lee JS, Bae ON. Potent Anti-inflammatory and Analgesic Actions of the Chloroform Extract of Dendropanax morbifera Mediated by the Nrf2/HO-1 Pathway. Biol Pharm Bull. 2016; 39(5):728–736.13. Jung HY, Chung TH, Hwang IK. Dendropanax morbifera Léveille extract ameliorates memory impairments and inflammatory responses in the hippocampus of streptozotocin-induced type 1 diabetic rats. Mol Cell Toxicol. 2016; 12(4):429–436.14. Yoo DY, Kim W, Lee CH, Shin BN, Nam SM, Choi JH, Won MH, Yoon YS, Hwang IK. Melatonin improves D-galactose-induced aging effects on behavior, neurogenesis, and lipid peroxidation in the mouse dentate gyrus via increasing pCREB expression. J Pineal Res. 2012; 52(1):21–28.15. Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. 2012; 18(1):82–97.16. Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, Sutherland RJ. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 2003; 13(12):1344–1351.17. Morris RGM. Spatial localization does not require the presence of local cues. Learn Motiv. 1981; 12(2):239–260.18. Yu Y, Bai F, Wang W, Liu Y, Yuan Q, Qu S, Zhang T, Tian G, Li S, Li D, Ren G. Fibroblast growth factor 21 protects mouse brain against D-galactose induced aging via suppression of oxidative stress response and advanced glycation end products formation. Pharmacol Biochem Behav. 2015; 133:122–131.19. Kim W, Yim HS, Yoo DY, Jung HY, Kim JW, Choi JH, Yoon YS, Kim DW, Hwang IK. Dendropanax morbifera Léveille extract ameliorates cadmium-induced impairment in memory and hippocampal neurogenesis in rats. BMC Complement Altern Med. 2016; 16(1):452.20. Kim JM, Park SK, Guo TJ, Kang JY, Ha JS, Lee DS, Lee U, Heo HJ. Anti-amnesic effect of Dendropanax morbifera via JNK signaling pathway on cognitive dysfunction in high-fat diet-induced diabetic mice. Behav Brain Res. 2016; 312:39–54.21. Kohman RA, Bhattacharya TK, Wojcik E, Rhodes JS. Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. J Neuroinflammation. 2013; 10:114.22. Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003; 3(1):23–35.23. Li Y, Liu L, Barger SW, Griffin WS. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 2003; 23(5):1605–1611.24. Gemma C, Bachstetter AD, Bickford PC. Neuron-Microglia Dialogue and Hippocampal Neurogenesis in the Aged Brain. Aging Dis. 2010; 1(3):232–244.25. Ye SM, Johnson RW. Regulation of interleukin-6 gene expression in brain of aged mice by nuclear factor kappaB. J Neuroimmunol. 2001; 117(1-2):87–96.26. Quintanilla RA, Orellana DI, González-Billault C, Maccioni RB. Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Exp Cell Res. 2004; 295(1):245–257.27. Bellinger FP, Madamba S, Siggins GR. Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993; 628(1-2):227–234.28. Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006; 99(4):1263–1272.29. Maher FO, Nolan Y, Lynch MA. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol Aging. 2005; 26(5):717–728.30. Clarke RM, Lyons A, O'Connell F, Deighan BF, Barry CE, Anyakoha NG, Nicolaou A, Lynch MA. A pivotal role for interleukin-4 in atorvastatin-associated neuroprotection in rat brain. J Biol Chem. 2008; 283(4):1808–1817.31. Lyons A, Griffin RJ, Costelloe CE, Clarke RM, Lynch MA. IL-4 attenuates the neuroinflammation induced by amyloid-beta in vivo and in vitro. J Neurochem. 2007; 101(3):771–781.32. Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001; 9(4):183–192.33. Perez SD, Du K, Rendeiro C, Wang L, Wu Q, Rubakhin SS, Vazhappilly R, Baxter JH, Sweedler JV, Rhodes JS. A unique combination of micronutrients rejuvenates cognitive performance in aged mice. Behav Brain Res. 2017; 320:97–112.34. Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009; 23(3):309–317.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antimicrobial, Antioxidant and Cytotoxic Activities of Dendropanax morbifera Léveille extract for mouthwash and denture cleaning solution
- Dendropanax morbifera Extract Protects Cardiomyocytes against Hypoxia/Reoxygenation Injury by Inhibition of Reactive Oxygen Species Generation and Calcium Perturbation
- First Report of Foliar Blight on Dendropanax morbifera Caused by Alternaria panax
- Induction of Hepatocellular Carcinoma Cell Cycle Arrest and Apoptosis by Dendropanax morbifera Leveille Leaf Extract via the PI3K/AKT/mTOR Pathway
- The efficacy and safety of Dendropanax morbifera leaf extract on the metabolic syndrome: a 12-week, placebo controlled, double blind, and randomized controlled trial