Nat Prod Sci.
2019 Jun;25(2):136-142. 10.20307/nps.2019.25.2.136.
Dendropanax morbifera Extract Protects Cardiomyocytes against Hypoxia/Reoxygenation Injury by Inhibition of Reactive Oxygen Species Generation and Calcium Perturbation
- Affiliations
-
- 1Depatment of Biomedical Sciences, Chosun University Graduate School, Gwangju 61452 Korea. hsong@chosun.ac.kr
- 2Cancer Mutation Research Center, Chosun University, Gwangju 61452 Korea.
- 3Department of Biochemistry and Molecular Biology, Chosun University School of Medicine, Gwangju 61452 Korea.
- KMID: 2452783
- DOI: http://doi.org/10.20307/nps.2019.25.2.136
Abstract
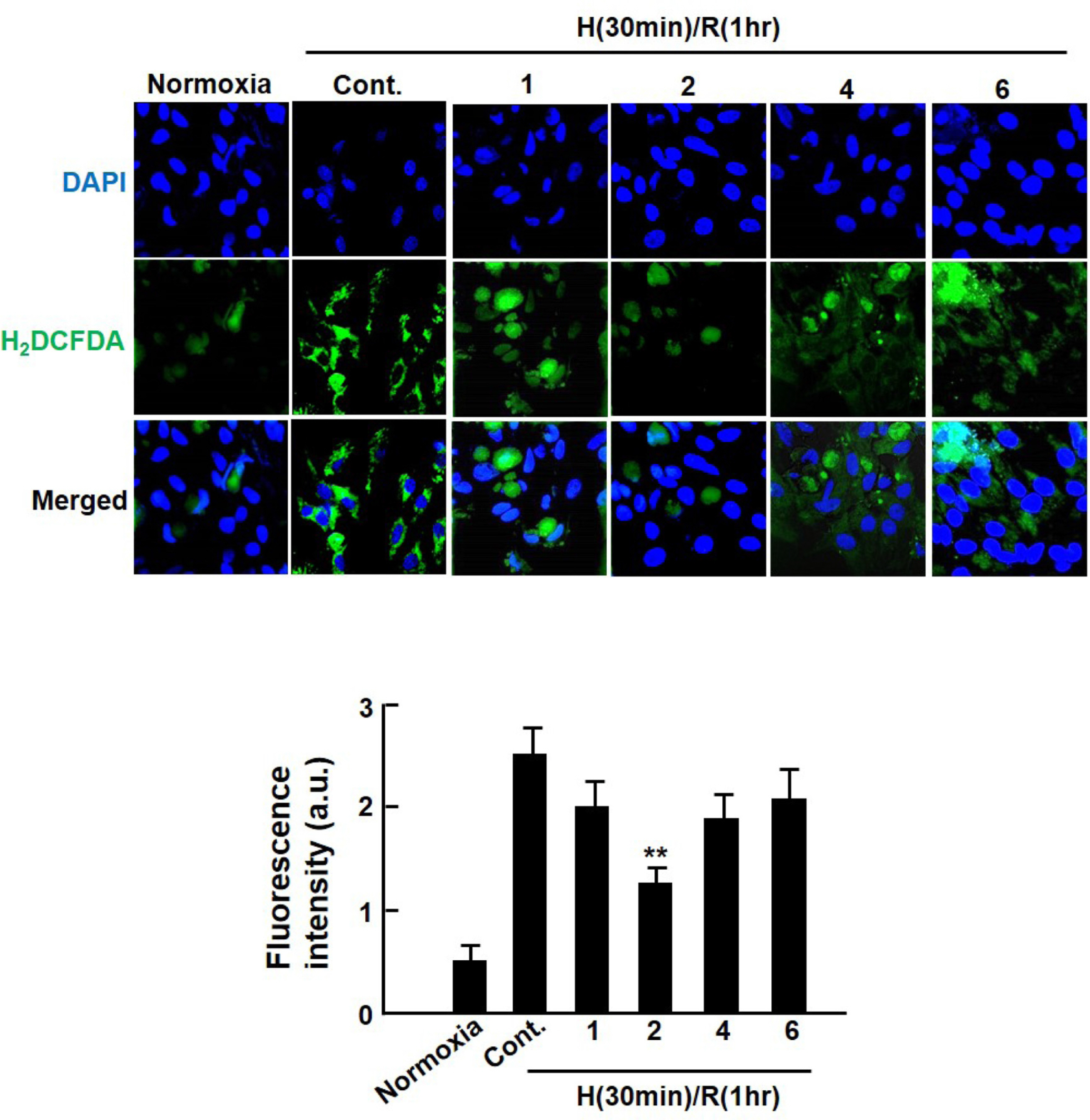
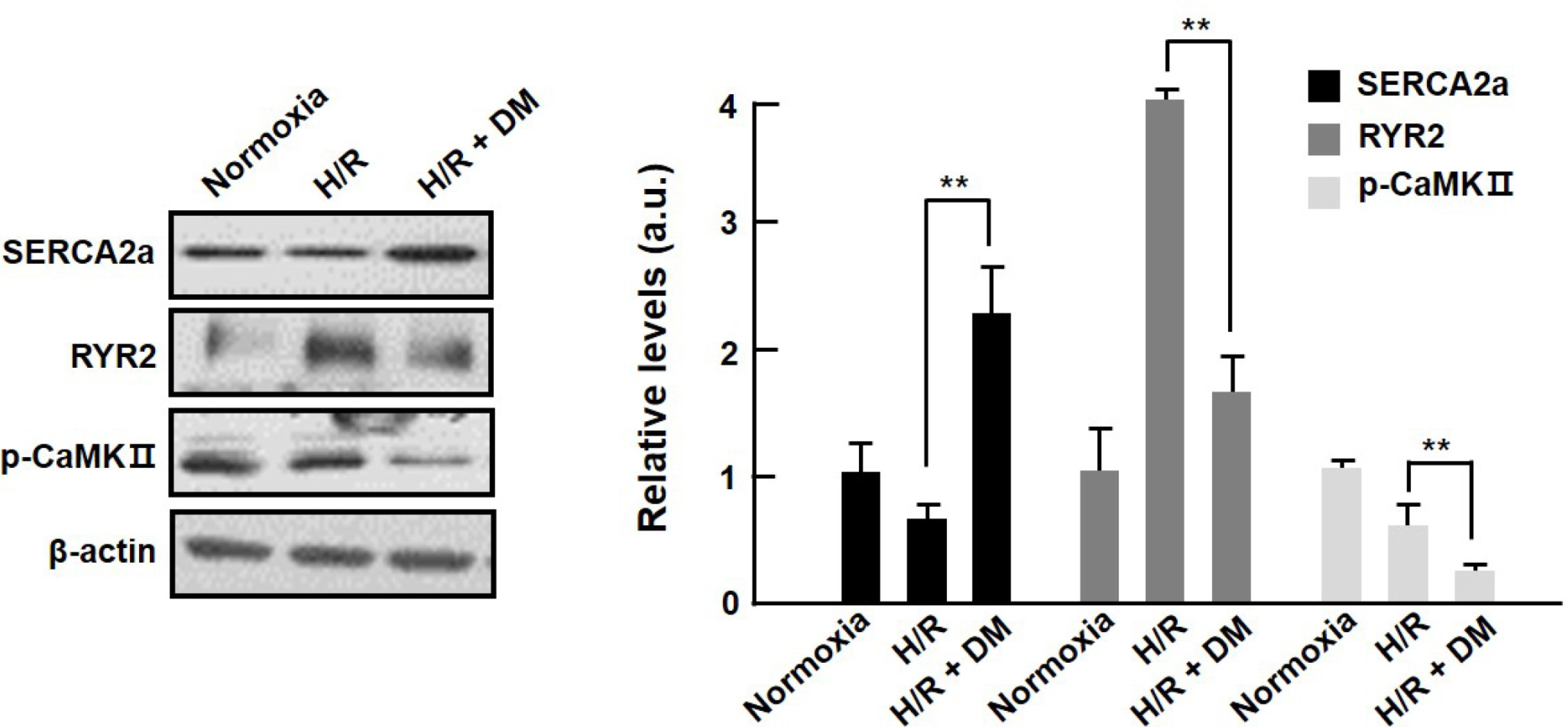
- Ischemia/reperfusion-induced myocardial injury is the main cause of acute myocardial infarction. Dendropanax morbifera Léveille has been used in traditional medicines for the treatment of various diseases such as headache, infectious diseases, and general debility. However, the effect of extract from D. morbifera (EDM) on myocardial ischemic injury is still unknown. In this study, the effects of EDM on neonatal rat cardiomyocytes with hypoxia/reoxygenation (H/R) injury were investigated. The viability of cardiomyocytes with H (30 min)/R (1 h) decreased; however, treatment with EDM significantly inhibited H/R injury-induced cardiomyocyte death. Further, we observed that reactive oxygen species (ROS) generation and intracellular calcium concentration (Ca²âºáµ¢) were significantly reduced in EDM-treated cardiomyocytes compared with that in H/R-injured positive control. In addition, western blotting results showed that EDM attenuated abnormal changes of RyR2 and SERCA2a genes in hypoxic cardiomyocytes. These results suggest that EDM ameliorates ROS generation and Ca²âºáµ¢ homeostasis to prevent dysregulation of calcium regulatory proteins in the heart, thereby exerting cardioprotective effects and reducing hypoxia-induced cardiomyocyte damage, which verifies the potential use of EDM as a new therapeutic agent for the treatment of myocardial ischemic injury.
MeSH Terms
Figure
Reference
-
(1). Hausenloy D. J., Yellon D. M. J.Clin. Invest. 2013; 123:92–100.(2). Cadenas S., Aragonés J., Landázuri M. O.Cardiovasc. Res. 2010; 88:219–228.(3). Sabharwal S. S., Schumacker P. T.Nat. Rev. Cancer. 2014; 14:709–721.(4). Sugamura K., Keaney J. F.Jr. Free Radic. Biol. Med. 2011; 51:978–992.(5). Viña J., Borras C., Abdelaziz K. M., Garcia-Valles R., Gomez-Cabrera M. C.Antioxid. Redox Signal. 2013; 19:779–787.(6). Duchen M. R., Verkhratsky A., Muallem S.Cell Calcium. 2008; 44:1–5.(7). Yu H. Y., Kim K. S., Lee Y. C., Moon H. I., Lee J. H.Evid. Based Complement. Alternat. Med. 2012; 2012:637512.(8). Park B. Y., Min B. S., Oh S. R., Kim J. H., Kim T. J., Kim D. H., Bae K. H., Lee H. K. J.Ethnopharmacol. 2004; 90:403–408.(9). Moon H. I.Hum. Exp. Toxicol. 2011; 30:870–875.(10). Chung I. M., Kim M. Y., Park W. H., Moon H. I.Pharmazie. 2009; 64:547–549.(11). Chung I. M., Kim M. Y., Park S. D., Park W. H., Moon H. I.Phytother. Res. 2009; 23:1634–1637.(12). Chung I. M., Song H. K., Kim S. J., Moon H. I.Phytother. Res. 2011; 25:784–786.(13). Akram M., Kim K. S., Kim E. S., Syed A. S., Kim C. Y., Lee J. S., Bae O. N.Biol. Pharm. Bull. 2016; 39:728–736.(14). Yu H. Y., Jin C. Y., Kim K. S., Lee Y. C., Park S. H., Kim G. Y., Kim W. J., Moon H. I., Choi Y. H., Lee J. H. J.Agric. Food Chem. 2012; 60:5400–5406.(15). Lee J. W., Kim K. S., An H. K., Kim C. H., Moon H. I., Lee Y. C.PLoS One. 2013; 8:e83611.(16). Jin C. Y., Yu H. Y., Park C., Han M. H., Hong S. H., Kim K. S., Lee Y. C., Chang Y. C., Cheong J., Moon S. K., Kim G. Y., Moon H. I., Kim W. J., Lee J. H., Choi Y. H.Int. J. Oncol. 2013; 43:1943–1950.(17). Vandergriff A. C., Hensley M. T., Cheng K. J.Vis. Exp. 2015; 98:e52726.(18). Buja L. M., Entman M. L.Circulation. 1998; 98:1355–1357.(18). Buja L. M., Entman M. L.Circulation. 1998; 98:1355–1357.(19). Griendling K. K., FitzGerald G. A.Circulation. 2003; 108:2034–2040.(20). Schriewer J. M., Peek C. B., Bass J., Schumacker P. T. J.Am. Heart Assoc. 2013; 2:e000159.(21). Barry W. H., Bridge J. H.Circulation. 1993; 87:1806–1815.(22). Choi K. M., Zhong Y., Hoit B. D., Grupp I. L., Hahn H., Dilly K. W., Guatimosim S., Lederer W. J., Matlib M. A. Am. J.Physiol. Heart Circ. Physiol. 2002; 283:H1398–H1408.(23). Boudina S., Abel E. D.Circulation. 2007; 115:3213–3223.(24). Trost S. U., Belke D. D., Bluhm W. F., Meyer M., Swanson E., Dillmann W. H.Diabetes. 2002; 51:1166–1171.(25). Kain V., Kumar S., Sitasawad S. L.Cardiovasc. Diabetol. 2011; 10:97.(26). Erickson J. R., Joiner M. L., Guan X., Kutschke W., Yang J., Oddis C. V., Bartlett R. K., Lowe J. S., O'Donnell S. E., Aykin-Burns N., Zimmerman M. C., Zimmerman K., Ham A. J., Weiss R. M., Spitz D. R., Shea M. A., Colbran R. J., Mohler P. J., Anderson M. E.Cell. 2008; 133:462–474.(27). Li Y. H., Chung H. C., Liu S. L., Chao T. H., Chen J. C.Int. Heart J. 2009; 50:207–220.(28). Kim W., Kim D. W., Yoo D. Y., Jung H. Y., Nam S. M., Kim J. W., Hong S. M., Kim D. W., Choi J. H., Moon S. M., Yoon Y. S., Hwang I. K.BMC. Complement. Altern. Med. 2014; 14:428.(29). Lee J. W., Park C., Han M. H., Hong S. H., Lee T. K., Lee S. H., Kim G. Y., Choi Y. H.Oncol. Rep. 2013; 30:1231–1238.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antimicrobial, Antioxidant and Cytotoxic Activities of Dendropanax morbifera Léveille extract for mouthwash and denture cleaning solution
- Nicorandil alleviated cardiac hypoxia/reoxygenation-induced cytotoxicity via upregulating ketone body metabolism and ACAT1 activity
- The Effect of Repetitive Hypoxia on Production of Lipid Peroxidation in Newborn Rat Brain
- Induction of Hepatocellular Carcinoma Cell Cycle Arrest and Apoptosis by Dendropanax morbifera Leveille Leaf Extract via the PI3K/AKT/mTOR Pathway
- The Influence of Propofol on Cell Viability after Reoxygenation in Rat Embryonic Heart H9c2 Cells