Korean J Physiol Pharmacol.
2017 Nov;21(6):599-607. 10.4196/kjpp.2017.21.6.599.
JPH203, a selective L-type amino acid transporter 1 inhibitor, induces mitochondria-dependent apoptosis in Saos2 human osteosarcoma cells
- Affiliations
-
- 1Department of Pharmacology, Chonnam National University Medical School, Gwangju 61469, Korea. jkkim57@jnu.ac.kr
- 2Department of Oral Physiology, Chosun University School of Dentistry, Gwangju 61452, Korea.
- 3Department of Bio-system Pharmacology, Osaka University Graduate School of Medicine, Osaka 565-0871, Japan.
- 4Department of Pharmaceutical Sciences, School of Pharmacy, University of Colorado Denver Anschutz Medical Campus, Aurora, Colorado 80045, USA.
- 5Department of Pharmacology and Toxicology, Kyorin University School of Medicine, Tokyo 181-8611, Japan.
- 6J-Pharma Co., Ltd., Yokohama, Kanagawa 230-0046, Japan.
- KMID: 2395254
- DOI: http://doi.org/10.4196/kjpp.2017.21.6.599
Abstract
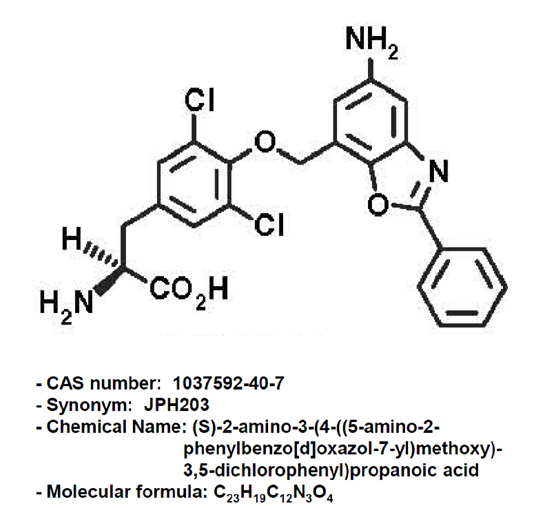
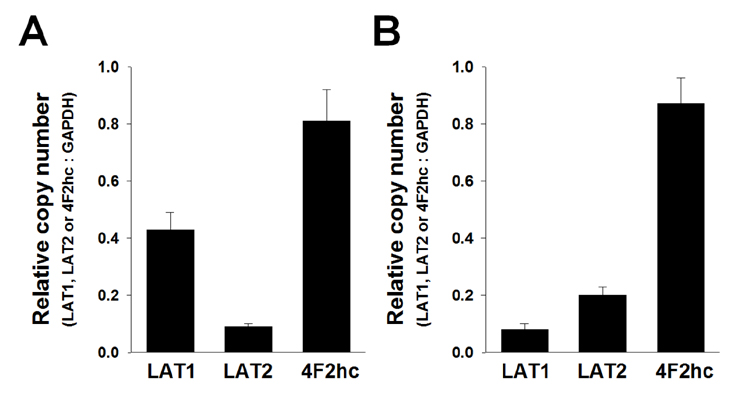
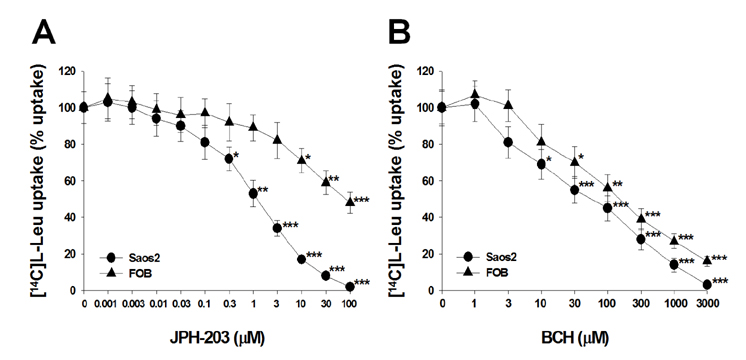
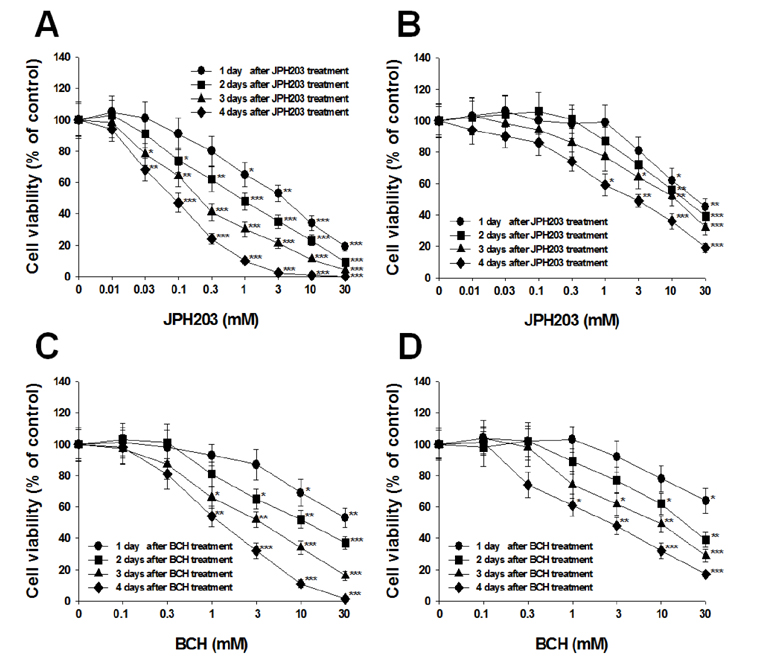
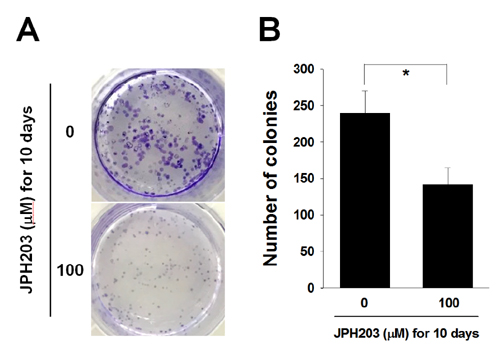
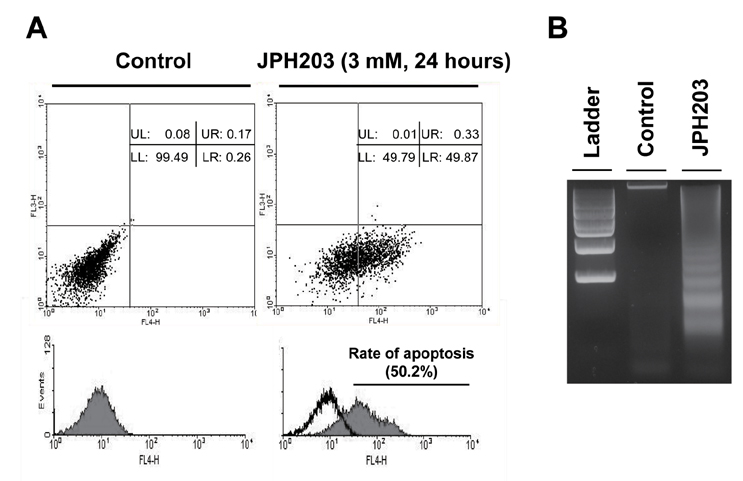
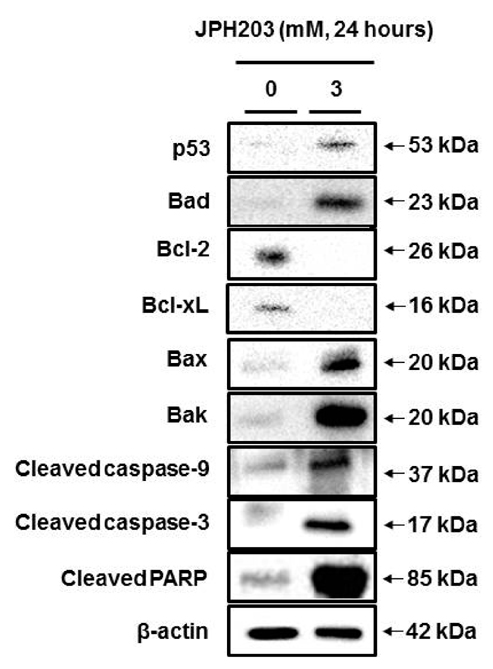
- Most normal cells express L-type amino acid transporter 2 (LAT2). However, L-type amino acid transporter 1 (LAT1) is highly expressed in many tumor cells and presumed to support their increased growth and proliferation. This study examined the effects of JPH203, a selective LAT1 inhibitor, on cell growth and its mechanism for cell death in Saos2 human osteosarcoma cells. FOB human osteoblastic cells and Saos2 cells expressed LAT1 and LAT2 together with their associating protein 4F2 heavy chain, but the expression of LAT2 in the Saos2 cells was especially weak. JPH203 and BCH, a non-selective L-type amino acid transporter inhibitor, potently inhibited L-leucine uptake in Saos2 cells. As expected, the intrinsic ability of JPH203 to inhibit L-leucine uptake was far more efficient than that of BCH in Saos2 cells. Likewise, JPH203 and BCH inhibited Saos2 cell growth with JPH203 being superior to BCH in this regard. Furthermore, JPH203 increased apoptosis rates and formed DNA ladder in Saos2 cells. Moreover, JPH203 activated the mitochondria-dependent apoptotic signaling pathway by upregulating pro-apoptotic factors, such as Bad, Bax, and Bak, and the active form of caspase-9, and downregulating anti-apoptotic factors, such as Bcl-2 and Bcl-xL. These results suggest that the inhibition of LAT1 activity via JPH203, which may act as a potential novel anti-cancer agent, leads to apoptosis mediated by the mitochondria-dependent intrinsic apoptotic signaling pathway by inducing the intracellular depletion of neutral amino acids essential for cell growth in Saos2 human osteosarcoma cells.
MeSH Terms
Figure
Reference
-
1. Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990; 70:43–77.2. McGivan JD, Pastor-Anglada M. Regulatory and molecular aspects of mammalian amino acid transport. Biochem J. 1994; 299:321–334.3. Kanai Y, Segawa H, Miyamoto Ki, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J Biol Chem. 1998; 273:23629–23632.4. Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999; 274:19745–19751.5. Babu E, Kanai Y, Chairoungdua A, Kim DK, Iribe Y, Tangtrongsup S, Jutabha P, Li Y, Ahmed N, Sakamoto S, Anzai N, Nagamori S, Endou H. Identification of a novel system L amino acid transporter structurally distinct from heterodimeric amino acid transporters. J Biol Chem. 2003; 278:43838–43845.6. Bodoy S, Martín L, Zorzano A, Palacín M, Estévez R, Bertran J. Identification of LAT4, a novel amino acid transporter with system L activity. J Biol Chem. 2005; 280:12002–12011.7. Yanagida O, Kanai Y, Chairoungdua A, Kim DK, Segawa H, Nii T, Cha SH, Matsuo H, Fukushima J, Fukasawa Y, Tani Y, Taketani Y, Uchino H, Kim JY, Inatomi J, Okayasu I, Miyamoto K, Takeda E, Goya T, Endou H. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta. 2001; 1514:291–302.8. Jin SE, Jin HE, Hong SS. Targeting L-type amino acid transporter 1 for anticancer therapy: clinical impact from diagnostics to therapeutics. Expert Opin Ther Targets. 2015; 19:1319–1337.9. Kim DK, Kanai Y, Choi HW, Tangtrongsup S, Chairoungdua A, Babu E, Tachampa K, Anzai N, Iribe Y, Endou H. Characterization of the system L amino acid transporter in T24 human bladder carcinoma cells. Biochim Biophys Acta. 2002; 1565:112–121.10. Kim CS, Moon IS, Park JH, Shin WC, Chun HS, Lee SY, Kook JK, Kim HJ, Park JC, Endou H, Kanai Y, Lee BK, Kim DK. Inhibition of L-type amino acid transporter modulates the expression of cell cycle regulatory factors in KB oral cancer cells. Biol Pharm Bull. 2010; 33:1117–1121.11. Kim CS, Cho SH, Chun HS, Lee SY, Endou H, Kanai Y, Kim DK. BCH, an inhibitor of system L amino acid transporters, induces apoptosis in cancer cells. Biol Pharm Bull. 2008; 31:1096–1100.12. Oda K, Hosoda N, Endo H, Saito K, Tsujihara K, Yamamura M, Sakata T, Anzai N, Wempe MF, Kanai Y, Endou H. L-type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer Sci. 2010; 101:173–179.13. Yun DW, Lee SA, Park MG, Kim JS, Yu SK, Park MR, Kim SG, Oh JS, Kim CS, Kim HJ, Kim JS, Chun HS, Kanai Y, Endou H, Wempe MF, Kim DK. JPH203, an L-type amino acid transporter 1-selective compound, induces apoptosis of YD-38 human oral cancer cells. J Pharmacol Sci. 2014; 124:208–217.14. Rosilio C, Nebout M, Imbert V, Griessinger E, Neffati Z, Benadiba J, Hagenbeek T, Spits H, Reverso J, Ambrosetti D, Michiels JF, Bailly-Maitre B, Endou H, Wempe MF, Peyron JF. L-type amino-acid transporter 1 (LAT1): a therapeutic target supporting growth and survival of T-cell lymphoblastic lymphoma/T-cell acute lymphoblastic leukemia. Leukemia. 2015; 29:1253–1266.15. Cormerais Y, Giuliano S, LeFloch R, Front B, Durivault J, Tambutté E, Massard PA, de la Ballina LR, Endou H, Wempe MF, Palacin M, Parks SK, Pouyssegur J. Genetic disruption of the multifunctional CD98/LAT1 complex demonstrates the key role of essential amino acid transport in the control of mTORC1 and tumor growth. Cancer Res. 2016; 76:4481–4492.16. Dai H, Huang Y, Li Y, Meng G, Wang Y, Guo QN. TSSC3 overexpression associates with growth inhibition, apoptosis induction and enhances chemotherapeutic effects in human osteosarcoma. Carcinogenesis. 2012; 33:30–40.17. Fuchs B, Pritchard DJ. Etiology of osteosarcoma. Clin Orthop Relat Res. 2002; (397):40–52.18. Koshi H, Sano T, Handa T, Yanagawa T, Saitou K, Nagamori S, Kanai Y, Takagishi K, Oyama T. L-type amino acid transporter-1 and CD98 expression in bone and soft tissue tumors. Pathol Int. 2015; 65:460–467.19. Kim SG, Kim HH, Kim HK, Kim CH, Chun HS, Kanai Y, Endou H, Kim DK. Differential expression and functional characterization of system L amino acid transporters in human normal osteoblast cells and osteogenic sarcoma cells. Anticancer Res. 2006; 26:1989–1996.20. Yoon JH, Kim YB, Kim MS, Park JC, Kook JK, Jung HM, Kim SG, Yoo H, Ko YM, Lee SH, Kim BY, Chun HS, Kanai Y, Endou H, Kim DK. Expression and functional characterization of the system L amino acid transporter in KB human oral epidermoid carcinoma cells. Cancer Lett. 2004; 205:215–226.21. Oberhammer FA, Hochegger K, Fröschl G, Tiefenbacher R, Pavelka M. Chromatin condensation during apoptosis is accompanied by degradation of lamin A+B, without enhanced activation of cdc2 kinase. J Cell Biol. 1994; 126:827–837.22. Seo YS, Yim MJ, Kim BH, Kang KR, Lee SY, Oh JS, You JS, Kim SG, Yu SJ, Lee GJ, Kim DK, Kim CS, Kim JS, Kim JS. Berberine-induced anticancer activities in FaDu head and neck squamous cell carcinoma cells. Oncol Rep. 2015; 34:3025–3034.23. Yu CS, Huang AC, Lai KC, Huang YP, Lin MW, Yang JS, Chung JG. Diallyl trisulfide induces apoptosis in human primary colorectal cancer cells. Oncol Rep. 2012; 28:949–954.24. Yang SJ, Lee SA, Park MG, Kim JS, Yu SK, Kim CS, Kim JS, Kim SG, Oh JS, Kim HJ, Chun HS, Kim YH, Kim DK. Induction of apoptosis by diphenyldifluoroketone in osteogenic sarcoma cells is associated with activation of caspases. Oncol Rep. 2014; 31:2286–2292.25. Fischer B, Coelho D, Dufour P, Bergerat JP, Denis JM, Gueulette J, Bischoff P. Caspase 8-mediated cleavage of the pro-apoptotic BCL-2 family member BID in p53-dependent apoptosis. Biochem Biophys Res Commun. 2003; 306:516–522.26. Ikner A, Ashkenazi A. TWEAK induces apoptosis through a death-signaling complex comprising receptor-interacting protein 1 (RIP1), Fas-associated death domain (FADD), and caspase-8. J Biol Chem. 2011; 286:21546–21554.27. Fisher DE. The p53 tumor suppressor: critical regulator of life & death in cancer. Apoptosis. 2001; 6:7–15.28. Lavin MF, Gueven N. The complexity of p53 stabilization and activation. Cell Death Differ. 2006; 13:941–950.29. Ryu DS, Lee HS, Lee GS, Lee DS. Effects of the ethylacetate extract of Orostachys japonicus on induction of apoptosis through the p53-mediated signaling pathway in human gastric cancer cells. Biol Pharm Bull. 2012; 35:660–665.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression and functional characterization of amino acid transport system L in Saos2 human osteogenic sarcoma cells
- Characteristics of Mitochondrial Events in Synthetic Bile Acids-induced Apoptosis of Human Osteosarcoma Cells
- Differential Sensitivity of Taxol-induced Apoptosis in U2OS and SaOS2 Osteogenic Sarcoma Cells
- Physiological, Pharmacological and Toxicological Implications of Heterodimeric Amino Acid Transporters
- Amino Acid Transporters as Potential Therapeutic Targets in Thyroid Cancer