Nutr Res Pract.
2015 Jun;9(3):219-226. 10.4162/nrp.2015.9.3.219.
Anti-inflammatory effect of enzymatic hydrolysates from Styela clava flesh tissue in lipopolysaccharide-stimulated RAW 264.7 macrophages and in vivo zebrafish model
- Affiliations
-
- 1Institute of Marine Biotechnology, Pukyong National University, Busan 608-737, Korea.
- 2Department of Marine Life Science, Jeju National University, 102 Jejudaehak-ro, Jeju-si, Jeju 690-756, Korea. youjinj@jejunu.ac.kr
- KMID: 2313833
- DOI: http://doi.org/10.4162/nrp.2015.9.3.219
Abstract
- BACKGROUND/OBJECTIVES
In this study, potential anti-inflammatory effect of enzymatic hydrolysates from Styela clava flesh tissue was assessed via nitric oxide (NO) production in lipopolysaccahride (LPS) induced RAW 264.7 macrophages and in vivo zebrafish model.
MATERIALS/METHODS
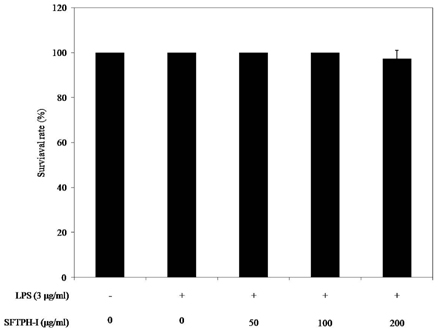
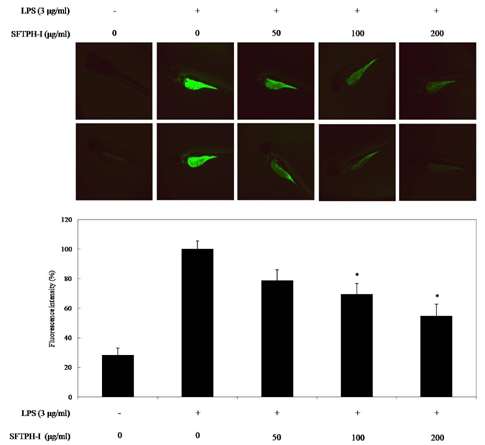
We investigated the ability of enzymatic hydrolysates from Styela clava flesh tissue to inhibit LPS-induced expression of pro-inflammatory mediators in RAW 264.7 macrophages, and the molecular mechanism through which this inhibition occurred. In addition, we evaluated anti-inflammatory effect of enzymatic hydrolysates against a LPS-exposed in in vivo zebrafish model.
RESULTS
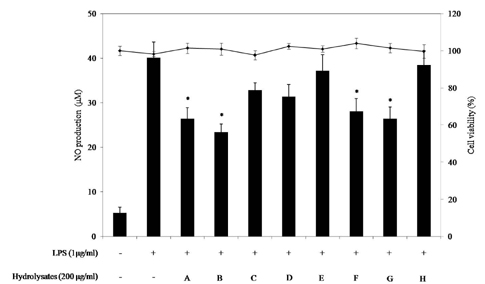
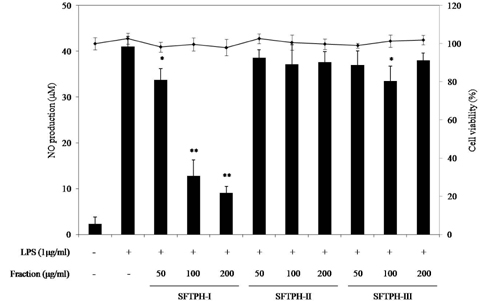
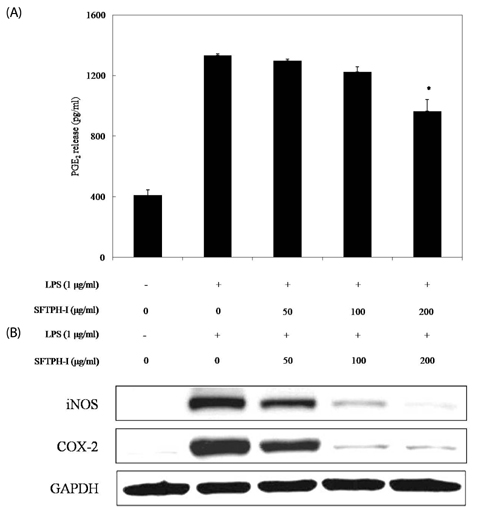
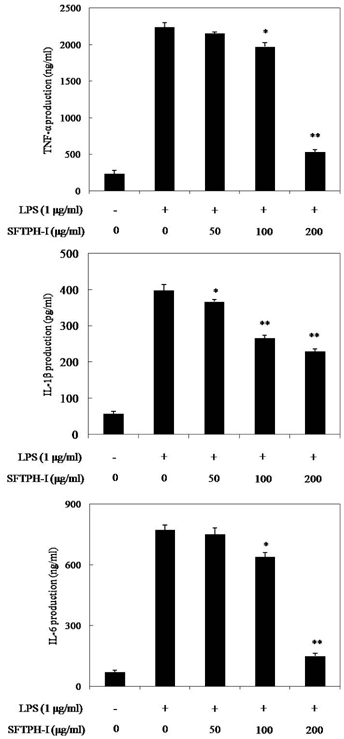
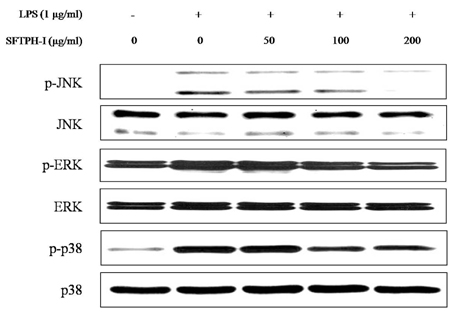
Among the enzymatic hydrolysates, Protamex-proteolytic hydrolysate exhibited the highest NO inhibitory effect and was fractionated into three ranges of molecular weight by using ultrafiltration (UF) membranes (MWCO 5 kDa and 10 kDa). The above 10 kDa fraction down-regulated LPS-induced expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), thereby reducing production of NO and prostaglandin E2 (PGE2) in LPS-activated RAW 264.7 macrophages. The above 10 kDa fraction suppressed LPS-induced production of pro-inflammatory cytokines, including interleukin (IL)-1beta, IL-6, and tumor necrosis factor (TNF)-alpha. In addition, the above 10 kDa fraction inhibited LPS-induced phosphorylation of extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinase (JNK), and p38. Furthermore, NO production in live zebrafish induced by LPS was reduced by addition of the above 10 kDa fraction from S. clava enzymatic hydrolysate.
CONCLUSION
The results of this study suggested that hydrolysates derived from S. clava flesh tissue would be new anti-inflammation materials in functional resources.
MeSH Terms
-
Cyclooxygenase 2
Cytokines
Dinoprostone
Extracellular Signal-Regulated MAP Kinases
Interleukin-6
Interleukins
JNK Mitogen-Activated Protein Kinases
Macrophages*
Membranes
Molecular Weight
Nitric Oxide
Nitric Oxide Synthase Type II
Phosphorylation
Tumor Necrosis Factor-alpha
Ultrafiltration
Zebrafish*
Cyclooxygenase 2
Cytokines
Dinoprostone
Extracellular Signal-Regulated MAP Kinases
Interleukin-6
Interleukins
JNK Mitogen-Activated Protein Kinases
Nitric Oxide
Nitric Oxide Synthase Type II
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Heo SJ, Yoon WJ, Kim KN, Ahn GN, Kang SM, Kang DH, Affan A, Oh C, Jung WK, Jeon YJ. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem Toxicol. 2010; 48:2045–2051.
Article2. Lee SJ, Kim EK, Kim YS, Hwang JW, Lee KH, Choi DK, Kang H, Moon SH, Jeon BT, Park PJ. Purification and characterization of a nitric oxide inhibitory peptide from Ruditapes philippinarum. Food Chem Toxicol. 2012; 50:1660–1666.
Article3. Kim SY, Jeong HJ, Kim DW, Kim MJ, An JJ, Sohn EJ, Kang HW, Shin MJ, Ahn EH, Kwon SW, Kim DS, Cho SW, Park J, Eum WS, Choi SY. Transduced PEP-1-FK506BP inhibits the inflammatory response in the Raw 264.7 cell and mouse models. Immunobiology. 2011; 216:771–781.
Article4. Schoonheim PJ, Chatzopoulou A, Schaaf MJ. The zebrafish as an in vivo model system for glucocorticoid resistance. Steroids. 2010; 75:918–925.
Article5. Ko SC, Cha SH, Heo SJ, Lee SH, Kang SM, Jeon YJ. Protective effect of Ecklonia cava on UVB-induced oxidative stress: in vitro and in vivo zebrafish model. J Appl Phycol. 2011; 23:697–708.
Article6. Alex D, Lam IK, Lin Z, Lee SM. Indirubin shows anti-angiogenic activity in an in vivo zebrafish model and an in vitro HUVEC model. J Ethnopharmacol. 2010; 131:242–247.
Article7. Park KH, Cho KH. A zebrafish model for the rapid evaluation of pro-oxidative and inflammatory death by lipopolysaccharide, oxidized low-density lipoproteins, and glycated high-density lipoproteins. Fish Shellfish Immunol. 2011; 31:904–910.
Article8. Jin S, Cho KH. Water extracts of cinnamon and clove exhibits potent inhibition of protein glycation and anti-atherosclerotic activity in vitro and in vivo hypolipidemic activity in zebrafish. Food Chem Toxicol. 2011; 49:1521–1529.
Article9. Lee DW, You DH, Yang EK, Jang IC, Bae MS, Jeon YJ, Kim SJ, Lee SC. Antioxidant and ACE inhibitory activities of Styela clava according to harvesting time. J Korean Soc Food Sci Nutr. 2010; 39:331–336.
Article10. Kim JJ, Kim SJ, Kim SH, Park HR, Lee SC. Antioxidant and anticancer activities of extracts from Styela clava according to the processing methods and solvents. J Korean Soc Food Sci Nutr. 2006; 35:278–283.
Article11. Kim JM, Park HR, Lee SC, Park E. Ethanol induced leucocytic and hepatic DNA strand breaks are prevented by Styela clava and Styela plicata supplementation in male SD rats. J Korean Soc Food Sci Nutr. 2007; 36:1271–1278.
Article12. Ko SC, Lee JK, Byun HG, Lee SC, Jeon YJ. Purification and characterization of angiotensin I-converting enzyme inhibitory peptide from enzymatic hydrolysates of Styela clava flesh tissue. Process Biochem. 2012; 47:34–40.
Article13. Ko SC, Kim DG, Han CH, Lee YJ, Lee JK, Byun HG, Lee SC, Park SJ, Lee DH, Jeon YJ. Nitric oxide-mediated vasorelaxation effects of anti-angiotensin I-converting enzyme (ACE) peptide from Styela clava flesh tissue and its anti-hypertensive effect in spontaneously hypertensive rats. Food Chem. 2012; 134:1141–1145.
Article14. Chen C, Chi YJ, Zhao MY, Xu W. Influence of degree of hydrolysis on functional properties, antioxidant and ACE inhibitory activities of egg white protein hydrolysate. Food Sci Biotechnol. 2012; 21:27–34.
Article15. Jeon YJ, Byun HG, Kim SK. Improvement of functional properties of cod frame protein hydrolysates using ultrafiltration membranes. Process Biochem. 1999; 35:471–478.
Article16. Ko SC, Kang M, Lee JK, Byun HG, Kim SK, Lee SC, Jeon BT, Park PJ, Jung WK, Jeon YJ. Effect of angiotensin I-converting enzyme (ACE) inhibitory peptide purified from enzymatic hydrolysates of Styela plicata. Eur Food Res Technol. 2011; 233:915–922.
Article17. Byun HG, Kim SK. Purification and characterization of angiotensin I converting enzyme (ACE) inhibitory peptides from Alaska pollack (Theragra chalcogramma) skin. Process Biochem. 2001; 36:1155–1162.
Article18. Cho JY, Baik KU, Jung JH, Park MH. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur J Pharmacol. 2000; 398:399–407.
Article19. Wijesinghe WA, Kim EA, Kang MC, Lee WW, Lee HS, Vairappan CS, Jeon YJ. Assessment of anti-inflammatory effect of 5β-hydroxypalisadin B isolated from red seaweed Laurencia snackeyi in zebrafish embryo in vivo model. Environ Toxicol Pharmacol. 2014; 37:110–117.
Article20. Samarakoon KW, Ko JY, Shah MM, Lee JH, Kang MC, Kwon ON, Lee JB, Jeon YJ. In vitro studies of anti-inflammatory and anticancer activities of organic solvent extracts from cultured marine microalgae. Algae. 2013; 28:111–119.
Article21. Kim HK, Cheon BS, Kim YH, Kim SY, Kim HP. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships. Biochem Pharmacol. 1999; 58:759–765.
Article22. Kim EK, Kim YS, Hwang JW, Kang SH, Choi DK, Lee KH, Lee JS, Moon SH, Jeon BT, Park PJ. Purification of a novel nitric oxide inhibitory peptide derived from enzymatic hydrolysates of Mytilus coruscus. Fish Shellfish Immunol. 2013; 34:1416–1420.
Article23. Lee SJ, Kim EK, Kim YS, Hwang JW, Lee KH, Choi DK, Kang H, Moon SH, Jeon BT, Park PJ. Purification and characterization of a nitric oxide inhibitory peptide from Ruditapes philippinarum. Food Chem Toxicol. 2012; 50:1660–1666.
Article24. Hsu KC, Li-Chan EC, Jao CL. Antiproliferative activity of peptides prepared from enzymatic hydrolysates of tuna dark muscle on human breast cancer cell line MCF-7. Food Chem. 2011; 126:617–622.
Article25. Chen J, Wang Y, Zhong Q, Wu Y, Xia W. Purification and characterization of a novel angiotensin-I converting enzyme (ACE) inhibitory peptide derived from enzymatic hydrolysate of grass carp protein. Peptides. 2012; 33:52–58.
Article26. Ko SC, Kim D, Jeon YJ. Protective effect of a novel antioxidative peptide purified from a marine Chlorella ellipsoidea protein against free radical-induced oxidative stress. Food Chem Toxicol. 2012; 50:2294–2302.
Article27. Bougatef A, Nedjar-Arroume N, Manni L, Ravallec R, Barkia A, Guillochon D, Nasri M. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 2010; 118:559–565.
Article28. Park HY, Han MH, Park C, Jin CY, Kim GY, Choi IW, Kim ND, Nam TJ, Kwon TK, Choi YH. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem Toxicol. 2011; 49:1745–1752.
Article29. Ahmad N, Chen LC, Gordon MA, Laskin JD, Laskin DL. Regulation of cyclooxygenase-2 by nitric oxide in activated hepatic macrophages during acute endotoxemia. J Leukoc Biol. 2002; 71:1005–1011.30. Höcherl K, Dreher F, Kurtz A, Bucher M. Cyclooxygenase-2 inhibition attenuates lipopolysaccharide-induced cardiovascular failure. Hypertension. 2002; 40:947–953.
Article31. Kim KN, Heo SJ, Yoon WJ, Kang SM, Ahn G, Yi TH, Jeon YJ. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur J Pharmacol. 2010; 649:369–375.
Article32. Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002; 109:Suppl. S81–S96.33. Lee HS, Ryu DS, Lee GS, Lee DS. Anti-inflammatory effects of dichloromethane fraction from Orostachys japonicus in RAW 264.7 cells: suppression of NF-κB activation and MAPK signaling. J Ethnopharmacol. 2012; 140:271–276.
Article34. Hseu YC, Wu FY, Wu JJ, Chen JY, Chang WH, Lu FJ, Lai YC, Yang HL. Anti-inflammatory potential of Antrodia Camphorata through inhibition of iNOS, COX-2 and cytokines via the NF-kappaB pathway. Int Immunopharmacol. 2005; 5:1914–1925.
Article35. Laskin DL, Pendino KJ. Macrophages and inflammatory mediators in tissue injury. Annu Rev Pharmacol Toxicol. 1995; 35:655–677.
Article36. Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012; 92:689–737.
Article37. Ajizian SJ, English BK, Meals EA. Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-gamma. J Infect Dis. 1999; 179:939–944.
Article38. Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005; 86:6–19.
Article39. Henry TR, Spitsbergen JM, Hornung MW, Abnet CC, Peterson RE. Early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio). Toxicol Appl Pharmacol. 1997; 142:56–68.
Article40. Vo TS, Ryu B, Kim SK. Purification of novel anti-inflammatory peptides from enzymatic hydrolysate of the edible microalgal Spirulina maxima. J Funct Foods. 2013; 5:1336–1346.
Article41. Ahn CB, Cho YS, Je JY. Purification and anti-inflammatory action of tripeptide from salmon pectoral fin byproduct protein hydrolysate. Food Chem. 2015; 168:151–156.
Article42. Kim EK, Kim YS, Hwang JW, Kang SH, Choi DK, Lee KH, Lee JS, Moon SH, Jeon BT, Park PJ. Purification of a novel nitric oxide inhibitory peptide derived from enzymatic hydrolysates of Mytilus coruscus. Fish Shellfish Immunol. 2013; 34:1416–1420.
Article43. Byun HG, Lee JK, Park HG, Jeon JK, Kim SK. Antioxidant peptides isolated from the marine rotifer, Brachionus rotundiformis. Process Biochem. 2009; 44:842–846.
Article44. Je JY, Qian ZJ, Byun HG, Kim SK. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007; 42:840–846.
Article45. Heaney RP. Factors influencing the measurement of bioavailability, taking calcium as a model. J Nutr. 2001; 131:1344S–1348S.
Article46. Phelan M, Aherne A, FitzGerald RJ, O'Brien NM. Casein-derived bioactive peptides: Biological effects, industrial uses, safety aspects and regulatory status. Int Dairy J. 2009; 19:643–654.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antihypertensive effect of an enzymatic hydrolysate from Styela clava flesh tissue in type 2 diabetic patients with hypertension
- The Anti-Inflammatory Effect of Pegmatite by in Vivo and in Vitro Study
- Suppression of Lipopolysaccharide-Induced Inflammatory and Oxidative Response by 5-Aminolevulinic Acid in RAW 264.7 Macrophages and Zebrafish Larvae
- YJI-7 Suppresses ROS Production and Expression of Inflammatory Mediators via Modulation of p38MAPK and JNK Signaling in RAW 264.7 Macrophages
- Anti-inflammatory effect of the water fraction from hawthorn fruit on LPS-stimulated RAW 264.7 cells