Nutr Res Pract.
2011 Apr;5(2):101-106.
Anti-inflammatory effect of the water fraction from hawthorn fruit on LPS-stimulated RAW 264.7 cells
- Affiliations
-
- 1College of Biomedical Science, Kangwon National University, 192-1 Hyoja-dong, Chuncheon-si, Gangwon 200-701, Korea. mhwang@kangwon.ac.kr
Abstract
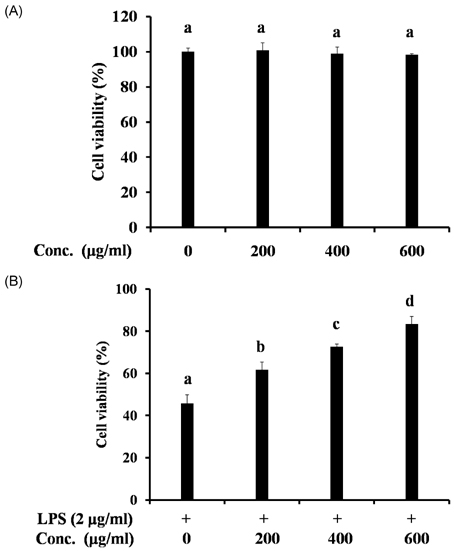
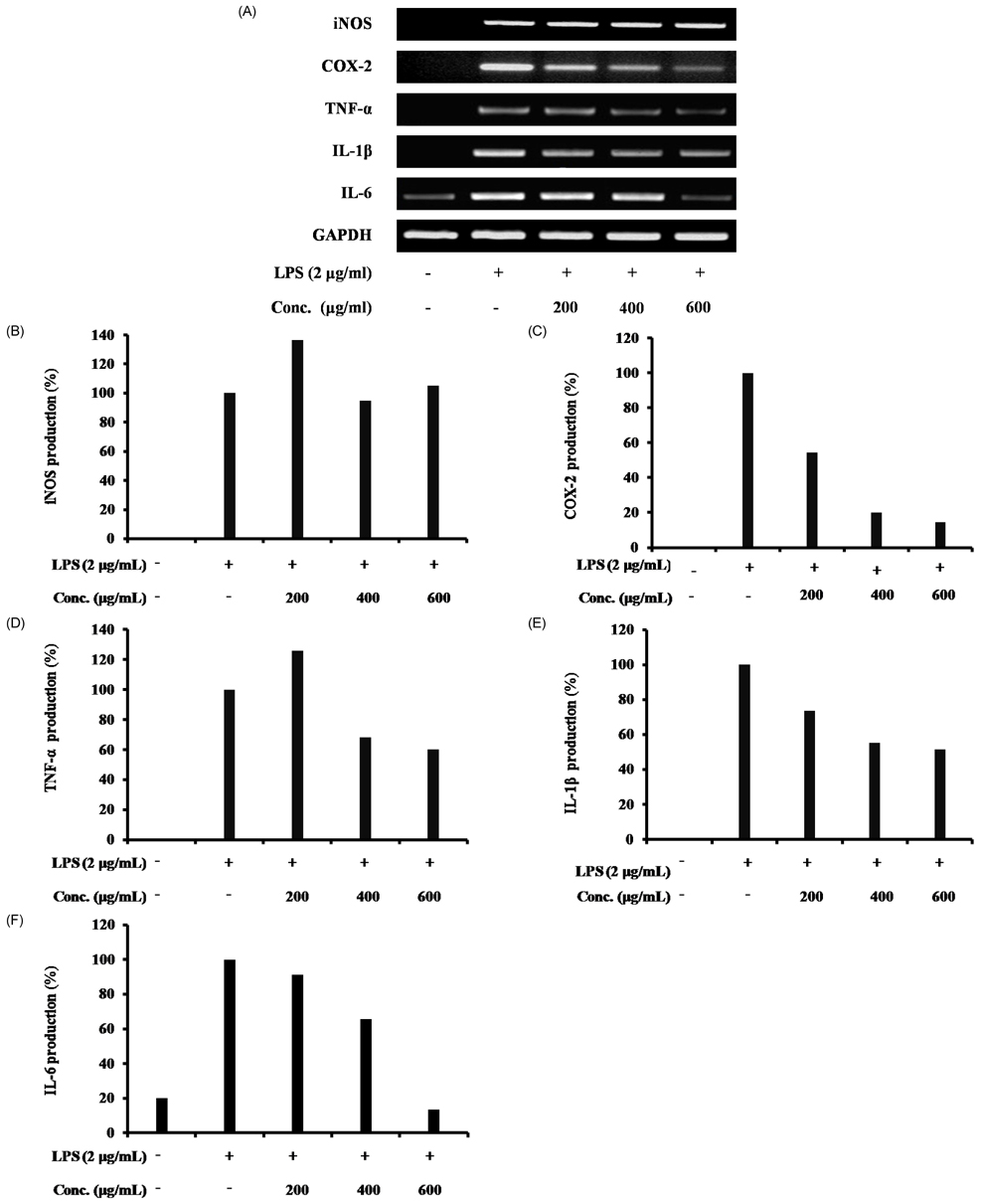
- The hawthorn fruit (Crataegus pinnatifida Bunge var. typica Schneider) is used as a traditional medicine in Korea. The objective of this study was to understand the mechanisms of the anti-inflammatory effects of the water fractionated portion of hawthorn fruit on a lipopolysaccharide (LPS)-stimulated RAW 264.7 cellular model. The level of nitric oxide (NO) production in the water fraction and LPS-treated RAW 264.7 cells were determined with an ELISA. The cytotoxicity of the water fraction and LPS was measured with an MTT assay. Expression of nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), tumor necrosis factor (TNF)-alpha, interleukin 6 (IL-6), and interleukin 1beta (IL-1beta) mRNA were analyzed with a reverse transcription polymerase chain reaction (RT-PCR). The water fraction of hawthorn fruit was determined to be safe and significantly inhibited NO production in LPS-stimulated RAW 264.7 cells and suppressed COX-2, TNF-alpha, IL-1beta, and IL-6 expression. The observed anti-inflammatory effects of the water fraction of hawthorn fruit might be attributed to the down-regulation of COX-2, TNF-alpha, IL-1beta, and IL-6 expression in LPS-stimulated RAW 264.7 cells.
Keyword
MeSH Terms
-
Crataegus
Cyclooxygenase 2
Down-Regulation
Enzyme-Linked Immunosorbent Assay
Fruit
Interleukin-1beta
Interleukin-6
Korea
Medicine, Traditional
Nitric Oxide
Nitric Oxide Synthase
Polymerase Chain Reaction
Reverse Transcription
RNA, Messenger
Tumor Necrosis Factor-alpha
Water
Cyclooxygenase 2
Interleukin-1beta
Interleukin-6
Nitric Oxide
Nitric Oxide Synthase
RNA, Messenger
Tumor Necrosis Factor-alpha
Water
Figure
Reference
-
1. Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol. 2007. 147:227–235.
Article2. Frostegård J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999. 145:33–43.
Article3. Jara LJ, Medina G, Vera-Lastra O, Amigo MC. Accelerated atherosclerosis, immune response and autoimmune rheumatic diseases. Autoimmun Rev. 2006. 5:195–201.
Article4. Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006. 124:823–835.
Article5. Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006. 236:13–23.
Article6. Walsh LJ. Mast cells and oral inflammation. Crit Rev Oral Biol Med. 2003. 14:188–198.
Article7. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005. 4:281–286.
Article8. Nicholas C, Batra S, Vargo MA, Voss OH, Gavrilin MA, Wewers MD, Guttridge DC, Grotewold E, Doseff AI. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-κB through the suppression of p65 phosphorylation. J Immunol. 2007. 179:7121–7127.
Article9. Hortelano S, Zeini M, Boscá L. Nitric oxide and resolution of inflammation. Methods Enzymol. 2002. 359:459–465.
Article10. Sacco RE, Waters WR, Rudolph KM, Drew ML. Comparative nitric oxide production by LPS-stimulated monocyte-derived macrophages from Ovis canadensis and Ovis aries. Comp Immunol Microbiol Infect Dis. 2006. 29:1–11.
Article11. Farley KS, Wang LF, Razavi HM, Law C, Rohan M, McCormack DG, Mehta S. Effects of macrophage inducible nitric oxide synthase in murine septic lung injury. Am J Physiol Lung Cell Mol Physiol. 2006. 290:L1164–L1172.
Article12. Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A. 1993. 90:7240–7244.
Article13. Yoon WJ, Ham YM, Kim KN, Park SY, Lee NH, Hyun CG, Lee WJ. Anti-inflammatory activity of brown alga Dictyota dichotoma in murine macrophage RAW 264.7 cells. J Med Plant Res. 2009. 3:1–8.14. Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest. 2006. 116:33–35.
Article15. Pryor WA. The antioxidant nutrients and disease prevention-what do we know and what do we need to find out? Am J Clin Nutr. 1991. 53:391S–393S.
Article16. Rigelsky JM, Sweet BV. Hawthorn: pharmacology and therapeutic uses. Am J Health Syst Pharm. 2002. 59:417–422.
Article17. Zhang Z, Ho WKK, Huang Y, James AE, Lam LW, Chen ZY. Hawthorn fruit is hypolipidemic in rabbits fed a high cholesterol diet. J Nutr. 2002. 132:5–10.
Article18. Froehlicher T, Hennebelle T, Martin-Nizard F, Cleenewerck P, Hilbert JL, Trotin F, Grec S. Phenolic profiles and antioxidative effects of hawthorn cell suspensions, fresh fruits, and medicinal dried parts. Food Chem. 2009. 115:897–903.
Article19. Kim KM, Choi JY, Yoo SE, Park MY, Lee BS, Ko TH, Sung SH, Shin HM, Park JE. HMCO5, herbal extract, inhibits NF-κB expression in lipopolysaccharide treated macrophages and reduces atherosclerotic lesions in cholesterol fed mice. J Ethnopharmacol. 2007. 114:316–324.
Article20. Ko SH, Choi SW, Ye SK, Yoo S, Kim HS, Chung MH. Comparison of anti-oxidant activities of seventy herbs that have been used in Korean traditional medicine. Nutr Res Pract. 2008. 2:143–151.
Article21. Park JH, Li C, Hu W, Wang MH. Antioxidant and free radical scavenging activity of different fractions from hawthorn fruit. J Food Sci Nutr. 2010. 15:44–50.
Article22. Li C, Son HJ, Huang C, Lee SK, Lohakare J, Wang MH. Comparison of Crataegus pinnatifida Bunge var. typica Schneider and C. pinnatifida Bunge fruits for antioxidant, anti-α-glucosidase, and anti-inflammatory activities. Food Sci Biotechnol. 2010. 19:769–775.
Article23. Sherman MP, Aeberhard EE, Wong VZ, Griscavage JM, Ignarro LJ. Pyrrolidine dithiocarbamate inhibits induction of nitric oxide synthase activity in rat alveolar macrophages. Biochem Biophys Res Commun. 1993. 191:1301–1308.
Article24. MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997. 15:323–350.
Article25. Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996. 271:C1424–C1437.
Article26. Li XA, Guo L, Asmis R, Nikolova-Karakashian M, Smart EJ. Scavenger receptor BI prevents nitric oxide-induced cytotoxicity and endotoxin-induced death. Circ Res. 2006. 98:e60–e65.
Article27. Jayakumar T, Thomas PA, Geraldine P. In-vitro antioxidant activities of an ethanolic extract of the oyster mushroom, Pleurotus ostreatus. IFSET. 2009. 10:228–234.
Article28. Boscá L, Zeini M, Través PG, Hortelano S. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology. 2005. 208:249–258.
Article29. Sarkar D, Saha P, Gamre S, Bhattacharjee S, Hariharan C, Ganguly S, Sen R, Mandal G, Chattopadhyay S, Majumdar S, Chatterjee M. Anti-inflammatory effect of allylpyrocatechol in LPS-induced macrophages is mediated by suppression of iNOS and COX-2 via the NF-κB pathway. Int Immunopharmacol. 2008. 8:1264–1271.
Article30. Hori M, Kita M, Torihashi S, Miyamoto S, Won KJ, Sato K, Ozaki H, Karaki H. Upregulation of iNOS by COX-2 in muscularis resident macrophage of rat intestine stimulated with LPS. Am J Physiol Gastrointest Liver Physiol. 2001. 280:G930–G938.
Article31. Li C, Han W, Wang MH. Antioxidant activity of hawthorn fruit in vitro. J Appl Biol Chem. 2010. 53:8–12.32. Perkins DJ, Kniss DA. Blockade of nitric oxide formation down-regulates cyclooxygenase-2 and decreases PGE2 biosynthesis in macrophages. J Leukoc Biol. 1999. 65:792–799.
Article33. Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol. 2006. 38:1654–1661.
Article34. Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Ann Rev Immunol. 1999. 17:331–367.
Article35. Aggarwal BB, Natarajan K. Tumor necrosis factors: developments during the last decade. Eur Cytokine Netw. 1996. 7:93–124.36. Kim JY, Park SJ, Yun KJ, Cho YW, Park HJ, Lee KT. Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-kB in RAW264.7 macrophages. Eur J Pharmacol. 2008. 584:175–184.
Article37. Jung WK, Choi I, Lee DY, Yea SS, Choi YH, Kim MM, Park SG, Seo SK, Lee SW, Lee CM, Park YM, Choi IW. Caffeic acid phenethyl ester protects mice from lethal endotoxin shock and inhibits lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in RAW 264.7 macrophages via the p38/ERK and NF-kB pathways. Int J Biochem Cell Biol. 2008. 40:2572–2582.
Article38. Cheenpracha S, Park EJ, Yoshida WY, Barit C, Wall M, Pezzuto JM, Chang LC. Potential anti-inflammatory phenolic glycosides from the medicinal plant Moringa oleifera fruits. Bioorg Med Chem. 2010. 18:6598–6602.
Article39. Mueller M, Hobiger S, Jungbauer A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010. 122:987–999.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory Activity of Cordyceps bassiana Extract on LPS-induced Inflammation in RAW 264.7 Cells by Suppressing NF-κB Activation
- Anti-inflammatory Activity of Standardized Fraction from Inula helenium L. via Suppression of NF-κB Pathway in RAW 264.7 Cells
- Anti-Inflammatory Effects of Chrysanthemum indicum Water Extract in RAW 264.7 Cell as a Whole Plant
- The Anti-inflammatory Effects of Water Extract from Cordyceps militaris in Murine Macrophage
- Lignans and Macrolides from the Leaves of Houttuynia cordata with Inhibitory Activity against NO Production in Murine Macrophage RAW 264.7 Cells