Lab Anim Res.
2014 Mar;30(1):8-13. 10.5625/lar.2014.30.1.8.
Ferulic acid prevents the injury-induced decrease of gamma-enolase expression in brain tissue and HT22 cells
- Affiliations
-
- 1Department of Anatomy, College of Veterinary Medicine, Research Institute of Life Science, Gyeongsang National University, Jinju, Korea. pokoh@gnu.ac.kr
- KMID: 2312114
- DOI: http://doi.org/10.5625/lar.2014.30.1.8
Abstract
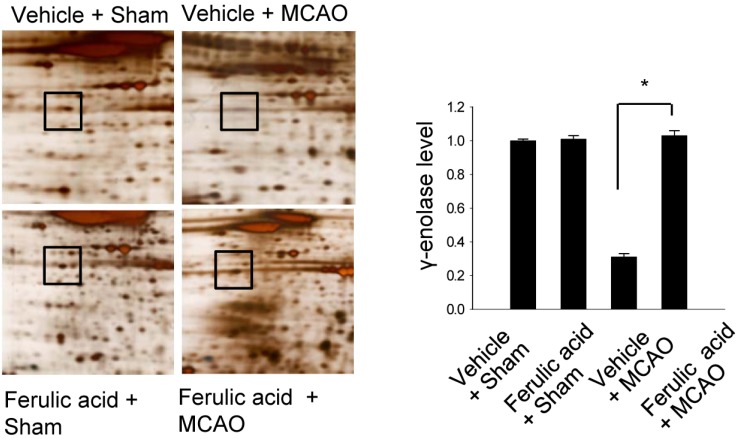
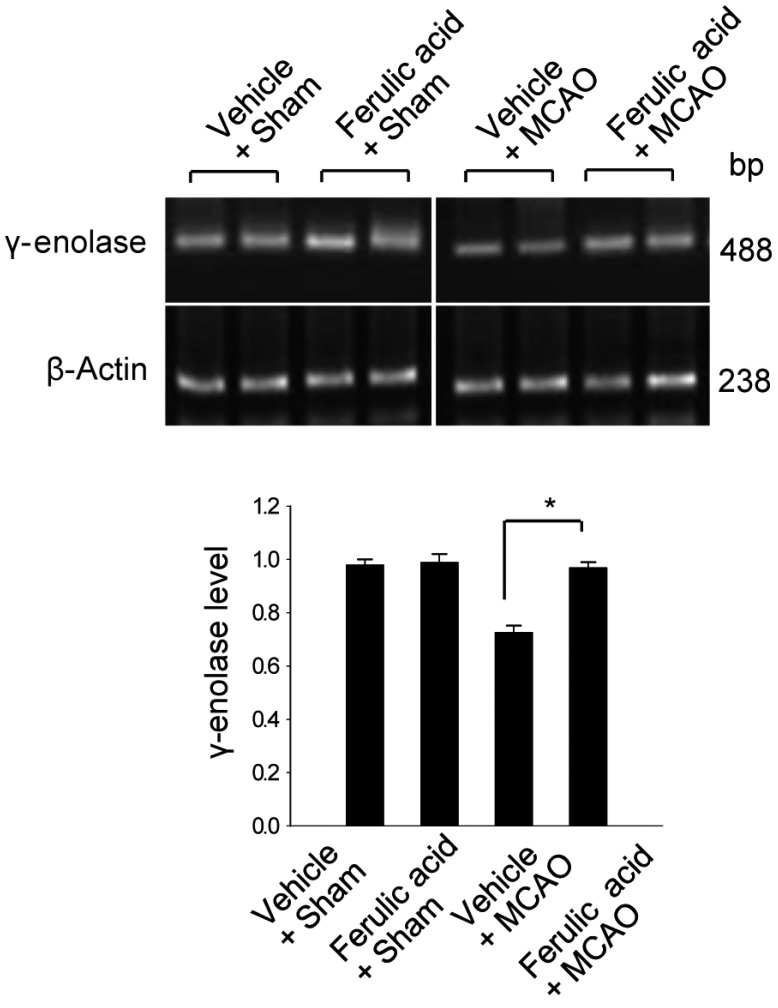
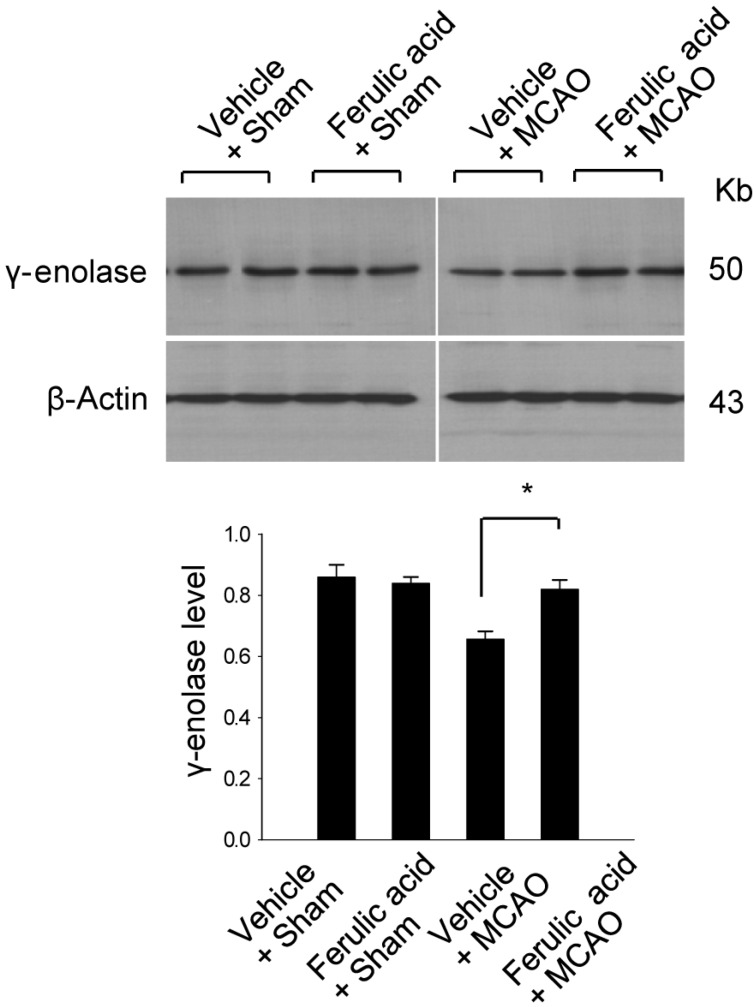
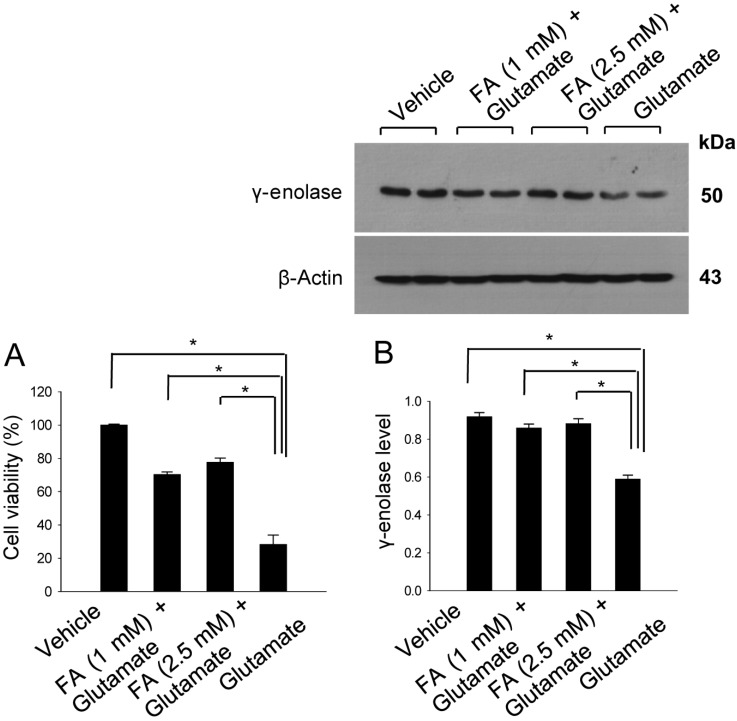
- Ferulic acid is known to act as a protective agent in cerebral ischemia through its anti-oxidant activity. gamma-Enolase is a neuron-specific enolase that also exerts a neuroprotective effect. Here, we investigated whether ferulic acid regulates the expression level of gamma-enolase in middle cerebral artery occlusion (MCAO)-induced brain injury and glutamate exposure-induced neuronal cell death. Adult male rats were treated with either vehicle or ferulic acid (100 mg/kg, i.v.) after MCAO and cerebral cortex tissues were collected 24 h after MCAO. Using a proteomics approach, we found that gamma-enolase expression was decreased in MCAO-injured animals treated with vehicle alone, whereas ferulic acid treatment attenuated this decrease. Reverse-transcription PCR and Western blot analyses confirmed that ferulic acid treatment prevented MCAO injury-induced decrease in gamma-enolase. Furthermore, in hippocampal-derived cell lines, glutamate exposure also decreased gamma-enolase expression and ferulic acid treatment attenuated this glutamate-induced decrease in gamma-enolase. These findings suggest that ferulic acid mediates a neuroprotective effect by attenuating injury-induced decreases of gamma-enolase expression in neuronal cells.
Keyword
MeSH Terms
-
Adult
Animals
Blotting, Western
Brain Injuries
Brain Ischemia
Brain*
Cell Death
Cell Line
Cerebral Cortex
Glutamic Acid
Humans
Infarction, Middle Cerebral Artery
Male
Neurons
Neuroprotective Agents
Phosphopyruvate Hydratase*
Polymerase Chain Reaction
Proteomics
Rats
Glutamic Acid
Neuroprotective Agents
Phosphopyruvate Hydratase
Figure
Cited by 1 articles
-
Curcumin attenuates the middle cerebral artery occlusion-induced reduction in γ-enolase expression in an animal model
Sang-Ah Gim, So-Ra Lee, Fawad-Ali Shah, Phil-Ok Koh
Lab Anim Res. 2015;31(4):198-203. doi: 10.5625/lar.2015.31.4.198.
Reference
-
1. Rider CC, Taylor CB. Enolase isoenzymes in rat tissues. Electrophoretic, chromatographic, immunological and kinetic properties. Biochim Biophys Acta. 1974; 365(1):285–300. PMID: 4413246.
Article2. Schmechel DE, Brightman MW, Marangos PJ. Neurons switch from non-neuronal enolase to neuron-specific enolase during differentiation. Brain Res. 1980; 190(1):195–214. PMID: 6769533.
Article3. Hattori T, Takei N, Mizuno Y, Kato K, Kohsaka S. Neurotrophic and neuroprotective effects of neuron-specific enolase on cultured neurons from embryonic rat brain. Neurosci Res. 1995; 21(3):191–198. PMID: 7753500.
Article4. Hafner A, Obermajer N, Kos J. γ-Enolase C-terminal peptide promotes cell survival and neurite outgrowth by activation of the PI3K/Akt and MAPK/ERK signalling pathways. Biochem J. 2012; 443(2):439–450. PMID: 22257123.
Article5. Cheng CY, Ho TY, Lee EJ, Su SY, Tang NY, Hsieh CL. Ferulic acid reduces cerebral infarct through its antioxidative and anti-inflammatory effects following transient focal cerebral ischemia in rats. Am J Chin Med. 2008; 36(6):1105–1119. PMID: 19051339.
Article6. Gim SA, Sung JH, Shah FA, Kim MO, Koh PO. Ferulic acid regulates the AKT/GSK-3β/CRMP-2 signaling pathway in a middle cerebral artery occlusion animal model. Lab Anim Res. 2013; 29(2):63–69. PMID: 23825478.
Article7. Koh PO. Ferulic acid modulates nitric oxide synthase expression in focal cerebral ischemia. Lab Anim Res. 2012; 28(4):273–278. PMID: 23326288.
Article8. Jin Y, Yan EZ, Fan Y, Guo XL, Zhao YJ, Zong ZH, Liu Z. Neuroprotection by sodium ferulate against glutamate-induced apoptosis is mediated by ERK and PI3 kinase pathways. Acta Pharmacol Sin. 2007; 28(12):1881–1890. PMID: 18031600.
Article9. Cheng CY, Su SY, Tang NY, Ho TY, Chiang SY, Hsieh CL. Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res. 2008; 1209:136–150. PMID: 18400211.
Article10. Ou L, Kong LY, Zhang XM, Niwa M. Oxidation of ferulic acid by Momordica charantia peroxidase and related anti-inflammation activity changes. Biol Pharm Bull. 2003; 26(11):1511–1516. PMID: 14600392.
Article11. Joshi G, Perluigi M, Sultana R, Agrippino R, Calabrese V, Butterfield DA. In vivo protection of synaptosomes by ferulic acid ethyl ester (FAEE) from oxidative stress mediated by 2,2-azobis(2-amidino-propane)dihydrochloride (AAPH) or Fe(2+)/H(2)O(2): insight into mechanisms of neuroprotection and relevance to oxidative stress-related neurodegenerative disorders. Neurochem Int. 2006; 48(4):318–327. PMID: 16386335.
Article12. Srinivasan M, Sudheer AR, Menon VP. Ferulic Acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr. 2007; 40(2):92–100. PMID: 18188410.
Article13. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989; 20(1):84–91. PMID: 2643202.
Article14. Sung JH, Kim MO, Koh PO. Proteomic identification of proteins differentially expressed by nicotinamide in focal cerebral ischemic injury. Neuroscience. 2011; 174:171–177. PMID: 21111033.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ferulic acid modulates nitric oxide synthase expression in focal cerebral ischemia
- Ferulic acid regulates the AKT/GSK-3beta/CRMP-2 signaling pathway in a middle cerebral artery occlusion animal model
- Curcumin attenuates the middle cerebral artery occlusion-induced reduction in gamma-enolase expression in an animal model
- Focal Cerebral Ischemia Induces Decrease of Astrocytic Phosphoprotein PEA-15 in Brain Tissue and HT22 Cells
- Combined Method of Neuronal Cell-Inducible Vector and Valproic Acid for Enhanced Gene Expression under Hypoxic Conditions