Lab Anim Res.
2012 Dec;28(4):273-278. 10.5625/lar.2012.28.4.273.
Ferulic acid modulates nitric oxide synthase expression in focal cerebral ischemia
- Affiliations
-
- 1Department of Anatomy, College of Veterinary Medicine, Research Institute of Life Science, Gyeongsang National University, Jinju, Korea. pokoh@gnu.ac.kr
- KMID: 1431411
- DOI: http://doi.org/10.5625/lar.2012.28.4.273
Abstract
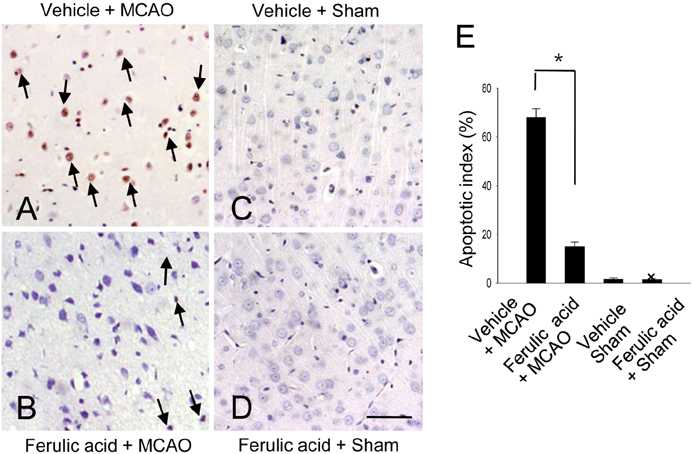
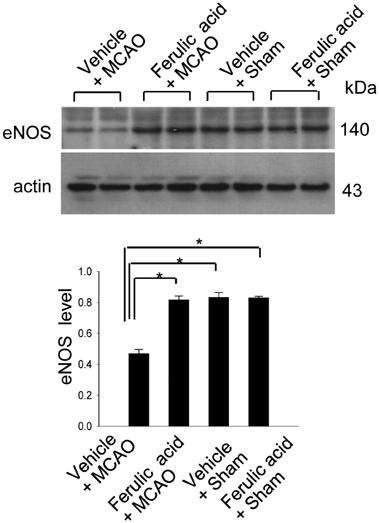
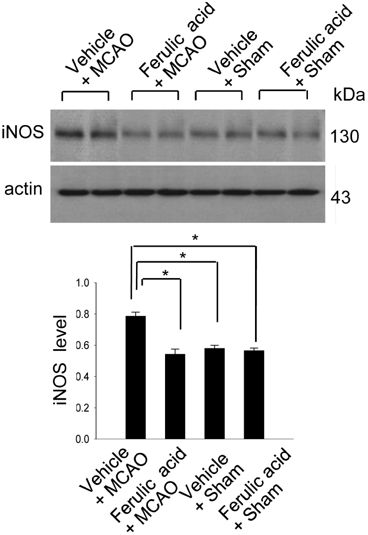
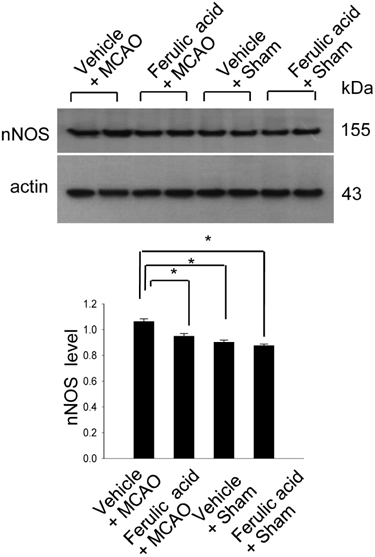
- Nitric oxide (NO) is generated by three different NO synthase (NOS) isoforms, endothelial NOS (eNOS), inducible NOS (iNOS), and neuronal NOS (nNOS). It is known that eNOS produces NO, which exerts a protective effect, while iNOS produces NO with neurotoxic effects. Ferulic acid preserves neuronal cells against from cerebral ischemia and glutamate-induced excitotoxicity. This study confirmed the neuroprotective effect of ferulic acid and investigated the levels of three NOS isoforms in focal cerebral ischemia with or without ferulic acid. Rats were immediately treated with ferulic acid (100 mg/kg, i.v.) after middle cerebral artery occlusion (MCAO). Brains tissues were collected at 24 h after the onset of occlusion. The expressions of these three isoforms in cerebral ischemia with ferulic acid were analyzed using Western blot technique. Ferulic acid treatment significantly decreases the number of TUNEL-positive cells in the cerebral cortex against MCAO injury. The levels of eNOS decreased in MCAO-operated animals, while ferulic acid treatment attenuated the MCAO-induced decrease of eNOS. However, iNOS and nNOS expression levels increased during MCAO, and ferulic acid prevented injury-induced increase of these isoforms. Thus, these findings suggest that the up- and down modulation of three isoforms by ferulic acid is associated with a neuroprotective mechanism.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Ferulic acid prevents the injury-induced decrease of γ-enolase expression in brain tissue and HT22 cells
Sang-A Gim, Phil-Ok Koh
Lab Anim Res. 2014;30(1):8-13. doi: 10.5625/lar.2014.30.1.8.
Reference
-
1. Cheng CY, Ho TY, Lee EJ, Su SY, Tang NY, Hsieh CL. Ferulic acid reduces cerebral infarct through its antioxidative and anti-inflammatory effects following transient focal cerebral ischemia in rats. Am J Chin Med. 2008. 36(6):1105–1119.2. Manosroi A, Chutoprapat R, Abe M, Manosroi W, Manosroi J. Anti-aging efficacy of topical formulations containing niosomes entrapped with rice bran bioactive compounds. Pharm Biol. 2012. 50(2):208–224.3. Cheng CY, Su SY, Tang NY, Ho TY, Chiang SY, Hsieh CL. Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res. 2008. 1209:136–150.4. Koh PO. Ferulic acid prevents the cerebral ischemic injury-induced decrease of Akt and Bad phosphorylation. Neurosci Lett. 2012. 507(2):156–160.5. Szabo C. Physiological and pathophysiological roles of nitric oxide in the central nervous system. Brain Res Bull. 1996. 41(3):131–141.6. Yun HY, Dawson VL, Dawson TM. Neurobiology of nitric oxide. Crit Rev Neurobiol. 1996. 10(3-4):291–316.7. Dalkara T, Moskowitz MA. The complex role of nitric oxide in the pathophysiology of focal cerebral ischemia. Brain Pathol. 1994. 4(1):49–57.8. Samdani AF, Dawson TM, Dawson VL. Nitric oxide synthase in models of focal ischemia. Stroke. 1997. 28(6):1283–1288.9. Davies SA, Stewart EJ, Huesmann GR, Skaer NJ, Maddrell SH, Tublitz NJ, Dow JA. Neuropeptide stimulation of the nitric oxide signaling pathway in Drosophila melanogaster Malpighian tubules. Am J Physiol. 1997. 273(2):R823–R827.10. Vane JR, Mitchell JA, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J, Willoughby DA. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci U S A. 1994. 91(6):2046–2050.11. Galea E, Reis DJ, Xu H, Feinstein DL. Transient expression of calcium-independent nitric oxide synthase in blood vessels during brain development. FASEB J. 1995. 9(15):1632–1637.12. Sparrow JR. Inducible nitric oxide synthase in the central nervous system. J Mol Neurosci. 1994-1995. 5(4):219–229.13. Hashiguchi A, Yano S, Morioka M, Hamada J, Ushio Y, Takeuchi Y, Fukunaga K. Up-regulation of endothelial nitric oxide synthase via phosphatidylinositol 3-kinase pathway contributes to ischemic tolerance in the CA1 subfield of gerbil hippocampus. J Cereb Blood Flow Metab. 2004. 24(3):271–279.14. Iadecola C, Zhang F, Casey R, Clark HB, Ross ME. Inducible nitric oxide synthase gene expression in vascular cells after transient focal cerebral ischemia. Stroke. 1996. 27(8):1373–1380.15. Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci. 1997. 17(23):9157–9164.16. Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, Moskowitz MA. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab. 1996. 16(5):981–987.17. Iadecola C, Pelligrino DA, Moskowitz MA, Lassen NA. Nitric oxide synthase inhibition and cerebrovascular regulation. J Cereb Blood Flow Metab. 1994. 14(2):175–192.18. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989. 20(1):84–91.19. Albrecht EW, Stegeman CA, Heeringa P, Henning RH, van Goor H. Protective role of endothelial nitric oxide synthase. J Pathol. 2003. 199(1):8–17.20. Marletta MA. Nitric oxide synthase: aspects concerning structure and catalysis. Cell. 1994. 78(6):927–930.21. Heeringa P, Steenbergen E, van Goor H. A protective role for endothelial nitric oxide synthase in glomerulonephritis. Kidney Int. 2002. 61(3):822–825.22. Dong TT, Zhao KJ, Gao QT, Ji ZN, Zhu TT, Li J, Duan R, Cheung AW, Tsim KW. Chemical and biological assessment of a chinese herbal decoction containing Radix Astragali and Radix Angelicae Sinensis: Determination of drug ratio in having optimized properties. J Agric Food Chem. 2006. 54(7):2767–2774.23. Hallenbeck JM, Dutka AJ. Background review and current concepts of reperfusion injury. Arch Neurol. 1990. 47(11):1245–1254.24. Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997. 17(10):1048–1056.25. Kuroda S, Siesjo BK. Reperfusion damage following focal ischemia: pathophysiology and therapeutic windows. Clin Neurosci. 1997. 4(4):199–212.26. Jander S, Schroeter M, Stoll G. Role of NMDA receptor signaling in the regulation of inflammatory gene expression after focal brain ischemia. J Neuroimmunol. 2000. 109(2):181–187.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nitric Oxide Synthase Inhibition Alters Extracellular Glutamate Concentration after Global Cerebral Ischemia
- Ferulic acid regulates the AKT/GSK-3beta/CRMP-2 signaling pathway in a middle cerebral artery occlusion animal model
- Different Expressions of eNOS and AQP4 after Focal Cerebral Ischemia in Rat: In Vasogenic Edema
- Effects of Hyperthermia on Neuronal Nitric Oxide Synthase Expression after Cerebral Ischemia in Gerbils
- Effect of Valproic Acid on Nitric Oxide and Nitric Oxide Synthase in Trabecular Meshwork Cell