Korean J Hematol.
2010 Sep;45(3):158-163. 10.5045/kjh.2010.45.3.158.
Induction of hypoxia-inducible factor-1alpha inhibits drug-induced apoptosis in the human leukemic cell line HL-60
- Affiliations
-
- 1Department of Pediatrics, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. hsahn@snu.ac.kr
- 2Laboratory of Immunology, School of Biological Science, Seoul National University, Seoul, Korea.
- 3BioResearch Institute, Daewoong Pharmaceutical Co., Ltd., Seoul, Korea.
- KMID: 2252055
- DOI: http://doi.org/10.5045/kjh.2010.45.3.158
Abstract
- BACKGROUND
Leukemic cells originate from hypoxic bone marrow, which protects them from anti-cancer drugs. Although many factors that cause drug resistance in leukemic cells have been studied, the effect of hypoxia on drug-induced apoptosis is still poorly understood.
METHODS
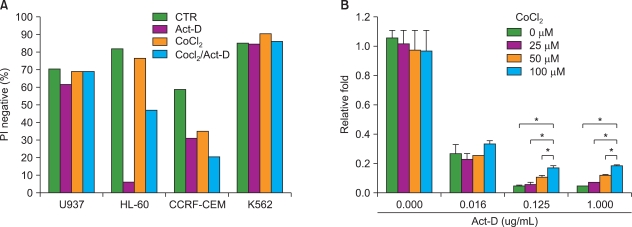
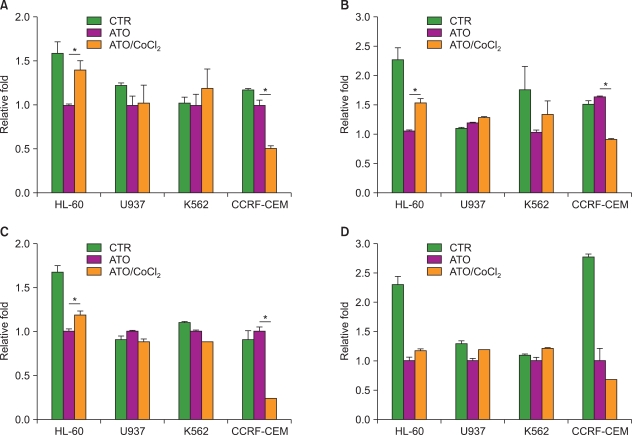
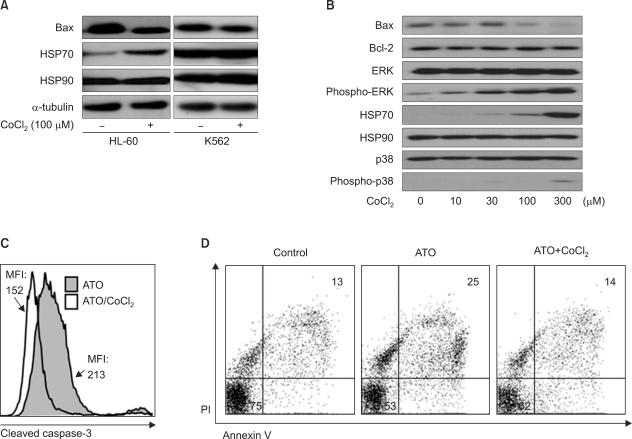
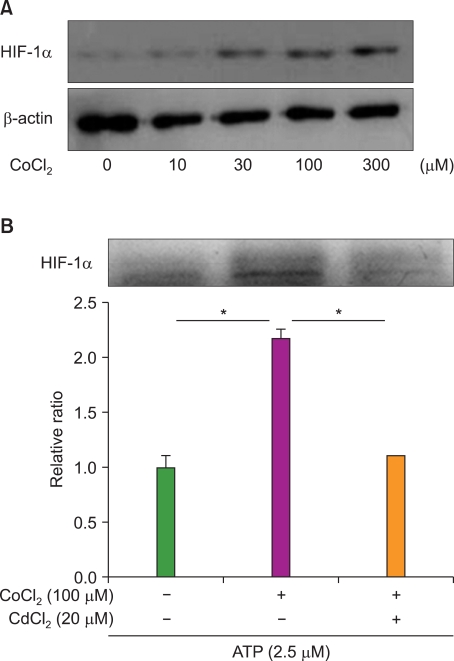
In this study, we examined the effect of hypoxia on anti-leukemic drug resistance in leukemic cell lines treated with cobalt chloride (CoCl2), a hypoxia-mimetic agent. Cellular proliferation was evaluated using the methyl thiazolyl tetrazolium (MTT) assay. Flow cytometry analysis and western blots were performed to investigate apoptosis-related proteins.
RESULTS
Unlike its previously known apoptotic effect, the expression of HIF-1alpha increased the survival rate of human promyelocytic leukemia HL-60 cells when these cells were exposed to anti-leukemic drugs; these effects were mediated by heat-shock protein HSP70 and the pro-apoptotic protein Bax.
CONCLUSION
These findings may provide new insights for understanding the mechanisms underlying hypoxia and for designing new therapeutic strategies for acute myeloid leukemia.
Keyword
MeSH Terms
Figure
Reference
-
1. Zhang TC, Cao EH, Li JF, Ma W, Qin JF. Induction of apoptosis and inhibition of human gastric cancer MGC-803 cell growth by arsenic trioxide. Eur J Cancer. 1999; 35:1258–1263. PMID: 10615238.
Article2. Park JW, Choi YJ, Jang MA, et al. Arsenic trioxide induces G2/M growth arrest and apoptosis after caspase-3 activation and bcl-2 phosphorylation in promonocytic U937 cells. Biochem Biophys Res Commun. 2001; 286:726–734. PMID: 11520058.
Article3. Uslu R, Sanli UA, Sezgin C, et al. Arsenic trioxide-mediated cytotoxicity and apoptosis in prostate and ovarian carcinoma cell lines. Clin Cancer Res. 2000; 6:4957–4964. PMID: 11156257.4. Shen ZY, Shen J, Cai WJ, Hong C, Zheng MH. The alteration of mitochondria is an early event of arsenic trioxide induced apoptosis in esophageal carcinoma cells. Int J Mol Med. 2000; 5:155–158. PMID: 10639594.
Article5. Maeda H, Hori S, Nishitoh H, et al. Tumor growth inhibition by arsenic trioxide (As2O3) in the orthotopic metastasis model of androgen-independent prostate cancer. Cancer Res. 2001; 61:5432–5440. PMID: 11454688.6. Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000; 14:1983–1991. PMID: 10950862.
Article7. Maxwell PH, Dachs GU, Gleadle JM, et al. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci USA. 1997; 94:8104–8109. PMID: 9223322.
Article8. Dachs GU, Patterson AV, Firth JD, et al. Targeting gene expression to hypoxic tumor cells. Nat Med. 1997; 3:515–520. PMID: 9142119.
Article9. Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002; 8(4 Suppl):S62–S67. PMID: 11927290.
Article10. Semenza GL. Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med. 2003; 54:17–28. PMID: 12359828.11. Yuan Y, Hilliard G, Ferguson T, Millhorn DE. Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. J Biol Chem. 2003; 278:15911–15916. PMID: 12606543.12. Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006; 12:1167–1174. PMID: 16998484.
Article13. Matsunaga T, Takemoto N, Sato T, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003; 9:1158–1165. PMID: 12897778.
Article14. Saitoh Y, Ouchida R, Miwa N. Bcl-2 prevents hypoxia/reoxygenation-induced cell death through suppressed generation of reactive oxygen species and upregulation of Bcl-2 proteins. J Cell Biochem. 2003; 90:914–924. PMID: 14624451.
Article15. Santore MT, McClintock DS, Lee VY, Budinger GR, Chandel NS. Anoxia-induced apoptosis occurs through a mitochondria-dependent pathway in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2002; 282:L727–L734. PMID: 11880298.16. Hussein D, Estlin EJ, Dive C, Makin GW. Chronic hypoxia promotes hypoxia-inducible factor-1alpha-dependent resistance to etoposide and vincristine in neuroblastoma cells. Mol Cancer Ther. 2006; 5:2241–2250. PMID: 16985058.17. Song X, Liu X, Chi W, et al. Hypoxia-induced resistance to cisplatin and doxorubicin in non-small cell lung cancer is inhibited by silencing of HIF-1alpha gene. Cancer Chemother Pharmacol. 2006; 58:776–784. PMID: 16532342.18. Walmsley SR, Print C, Farahi N, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005; 201:105–115. PMID: 15630139.19. Li Q, Sato EF, Zhu X, Inoue M. A simultaneous release of SOD1 with cytochrome c regulates mitochondria-dependent apoptosis. Mol Cell Biochem. 2009; 322:151–159. PMID: 19002561.
Article20. Khalil S, Luciano J, Chen W, Liu AY. Dynamic regulation and involvement of the heat shock transcriptional response in arsenic carcinogenesis. J Cell Physiol. 2006; 207:562–569. PMID: 16447264.
Article21. Ramos AM, Aller P. Quercetin decreases intracellular GSH content and potentiates the apoptotic action of the antileukemic drug arsenic trioxide in human leukemia cell lines. Biochem Pharmacol. 2008; 75:1912–1923. PMID: 18359480.
Article22. Chun YS, Choi E, Kim GT, et al. Cadmium blocks hypoxia-inducible factor (HIF)-1-mediated response to hypoxia by stimulating the proteasome-dependent degradation of HIF-1alpha. Eur J Biochem. 2000; 267:4198–4204. PMID: 10866824.23. Seo YJ, Koh SH, Kang HJ, Shin HY, Jeong G, Ahn HS. Hypoxia inhibits the SDF-1-dependent migration of human leukemic cell line HL-60 via blocking of Akt activation. Biochem Biophys Res Commun. 2007; 364:388–394. PMID: 17950696.
Article25. Takahashi Y, Karbowski M, Yamaguchi H, et al. Loss of Bif-1 suppresses Bax/Bak conformational change and mitochondrial apoptosis. Mol Cell Biol. 2005; 25:9369–9382. PMID: 16227588.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of Hypoxia Inducible Factor-1alpha by Desferrioxamine Induces Radioresistance in Mouse Hepatoma Cell Line
- The Effect of HIF-1alpha siRNA on Growth and Chemosensitivity of Mia-paca Cell Line
- Blockade of VEGFR-1 and VEGFR-2 Enhances Paclitaxel Sensitivity in Gastric Cancer Cells
- Effects of the knockdown of hypoxia inducible factor-1alpha expression by adenovirus-mediated shRNA on angiogenesis and tumor growth in hepatocellular carcinoma cell lines
- Sonication Induces Apoptosis in HL-60 Cells