Yonsei Med J.
2013 Mar;54(2):374-380. 10.3349/ymj.2013.54.2.374.
Blockade of VEGFR-1 and VEGFR-2 Enhances Paclitaxel Sensitivity in Gastric Cancer Cells
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Internal Medicine, Chonnam National University Hwasun Hospital, Hwasun, Korea. ijchung@chonnam.ac.kr
- KMID: 1503899
- DOI: http://doi.org/10.3349/ymj.2013.54.2.374
Abstract
- PURPOSE
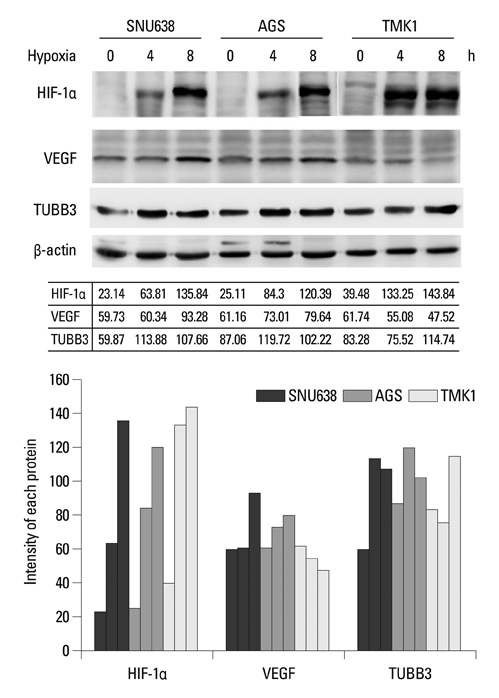
Hypoxia-inducible factor-1alpha (HIF-1alpha) increases transcription of the vascular endothelial growth factor (VEGF) gene. Inhibition of VEGF abolishes VEGF mediated induction of HIF-1alpha. Recent reports suggested that HIF-1alpha also mediated the induction of class III beta-tubulin (TUBB3) in hypoxia. TUBB3 confers resistance to taxanes. Inhibition of VEGF may decrease the expression of HIF-1alpha and TUBB3. This study was undertaken to investigate the roles of vascular endothelial growth factor receptor (VEGFR) in gastric cancer cell behavior and to identify methods to overcome paclitaxel resistance in vitro.
MATERIALS AND METHODS
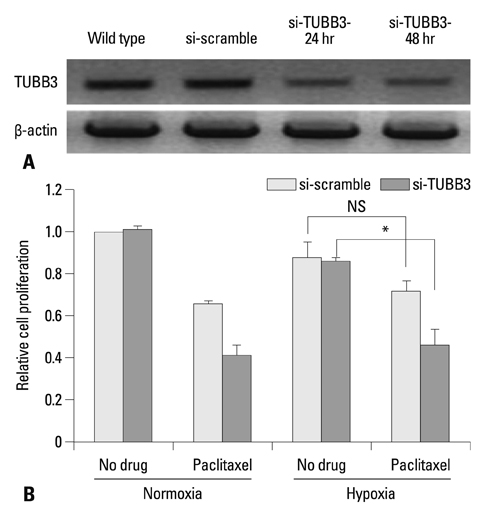
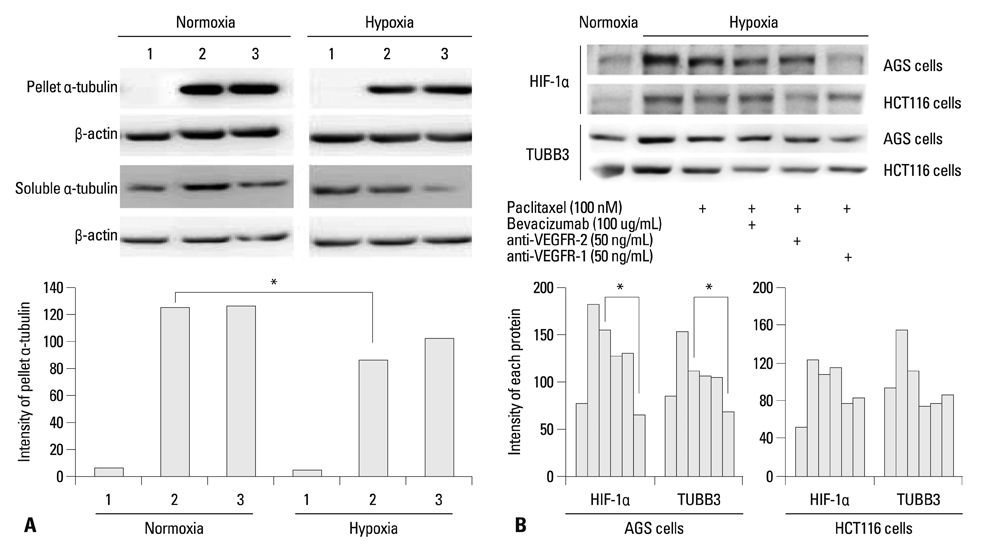
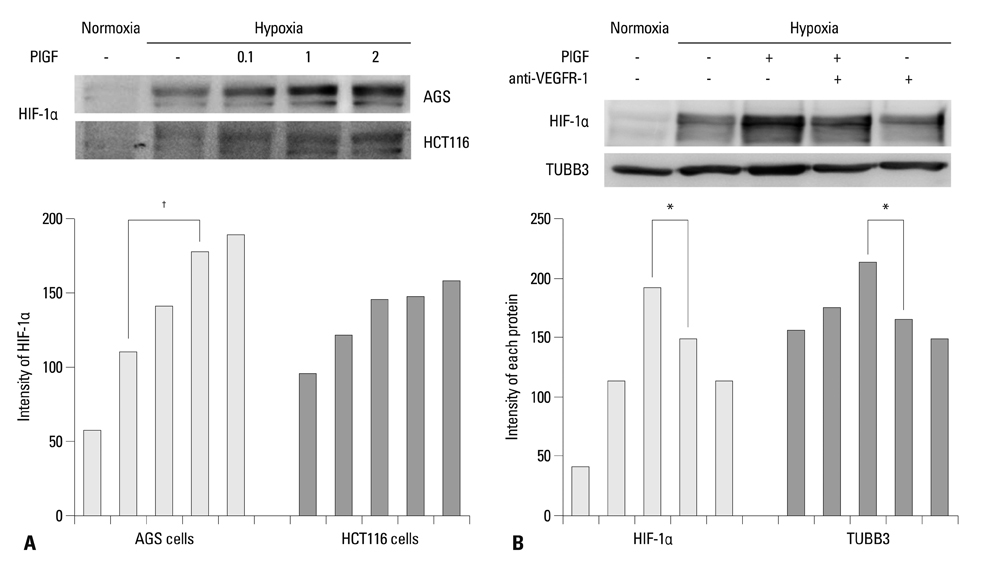
The protein expression levels of HIF-1alpha and TUBB3 were measured in human gastric cancer cell lines (AGS) under normoxic and hypoxic conditions. The relationship between TUBB3 and paclitaxel resistance was assessed with small interfering TUBB3 RNA. AGS cells were treated with anti-VEGFR-1, anti-VEGFR-2, placental growth factor (PlGF), bevacizuamb, and paclitaxel.
RESULTS
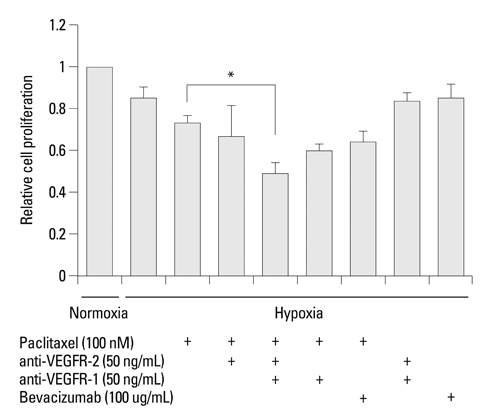
Hypoxia induced paclitaxel resistance was decreased by knockdown of TUBB3. Induction of HIF-1alpha and TUBB3 in AGS is VEGFR-1 mediated and PlGF dependent. Hypoxia-dependent upregulation of HIF-1alpha and TUBB3 was reduced in response to paclitaxel treatment. Expressions of HIF-1alpha and TUBB3 were most decreased when AGS cells were treated with a combination of paclitaxel and anti-VEGFR-1. AGS cell cytotoxicity was most increased in response to paclitaxel, anti-VEGFR-1, and anti-VEGFR-2.
CONCLUSION
We suggest that blockade of VEGFR-1 and VEGFR-2 enhances paclitaxel sensitivity in TUBB3-expressing gastric cancer cells.
Keyword
MeSH Terms
-
Angiogenesis Inhibitors/pharmacology
Antibodies, Monoclonal, Humanized/pharmacology
Antineoplastic Agents, Phytogenic/*pharmacology
Cell Hypoxia
Cell Line, Tumor
*Drug Resistance, Neoplasm
Gene Expression Regulation, Neoplastic/drug effects
Gene Knockdown Techniques
Humans
Hypoxia-Inducible Factor 1, alpha Subunit/metabolism
Paclitaxel/*pharmacology
Pregnancy Proteins/pharmacology
Stomach Neoplasms/drug therapy/genetics
Tubulin/genetics/metabolism
Vascular Endothelial Growth Factor Receptor-1/antagonists & inhibitors/*physiology
Vascular Endothelial Growth Factor Receptor-2/antagonists & inhibitors/*physiology
Angiogenesis Inhibitors
Antibodies, Monoclonal, Humanized
Antineoplastic Agents, Phytogenic
Hypoxia-Inducible Factor 1, alpha Subunit
Pregnancy Proteins
Tubulin
Paclitaxel
Vascular Endothelial Growth Factor Receptor-1
Vascular Endothelial Growth Factor Receptor-2
Figure
Reference
-
1. Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003. 14:Suppl 2. ii31–ii36.
Article2. Won YJ, Sung J, Jung KW, Kong HJ, Park S, Shin HR, et al. Nationwide cancer incidence in Korea, 2003-2005. Cancer Res Treat. 2009. 41:122–131.
Article3. Hruban RH, Yardley JH, Donehower RC, Boitnott JK. Taxol toxicity. Epithelial necrosis in the gastrointestinal tract associated with polymerized microtubule accumulation and mitotic arrest. Cancer. 1989. 63:1944–1950.
Article4. Ajani JA, Fodor MB, Tjulandin SA, Moiseyenko VM, Chao Y, Cabral Filho S, et al. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol. 2005. 23:5660–5667.
Article5. Constenla M, Garcia-Arroyo R, Lorenzo I, Carrete N, Campos B, Palacios P. Docetaxel, 5-fluorouracil, and leucovorin as treatment for advanced gastric cancer: results of a phase II study. Gastric Cancer. 2002. 5:142–147.
Article6. Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, et al. Paclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer. 1999. 85:295–301.
Article7. Murad AM, Petroianu A, Guimaraes RC, Aragao BC, Cabral LO, Scalabrini-Neto AO. Phase II trial of the combination of paclitaxel and 5-fluorouracil in the treatment of advanced gastric cancer: a novel, safe, and effective regimen. Am J Clin Oncol. 1999. 22:580–586.
Article8. Park SH, Lee WK, Chung M, Lee Y, Han SH, Bang SM, et al. Paclitaxel versus docetaxel for advanced gastric cancer: a randomized phase II trial in combination with infusional 5-fluorouracil. Anticancer Drugs. 2006. 17:225–229.
Article9. Roth AD, Maibach R, Martinelli G, Fazio N, Aapro MS, Pagani O, et al. Swiss Group for Clinical Cancer Research (SAKK), and the European Institute of Oncology (EIO). Docetaxel (Taxotere)-cisplatin (TC): an effective drug combination in gastric carcinoma. Ann Oncol. 2000. 11:301–306.
Article10. Roth AD, Fazio N, Stupp R, Falk S, Bernhard J, Saletti P, et al. Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: a randomized phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2007. 25:3217–3223.
Article11. Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006. 24:4991–4997.
Article12. Banerjee A. Increased levels of tyrosinated alpha-, beta(III)-, and beta(IV)-tubulin isotypes in paclitaxel-resistant MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2002. 293:598–601.
Article13. Kavallaris M, Kuo DY, Burkhart CA, Regl DL, Norris MD, Haber M, et al. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest. 1997. 100:1282–1293.
Article14. Kavallaris M, Burkhart CA, Horwitz SB. Antisense oligonucleotides to class III beta-tubulin sensitize drug-resistant cells to Taxol. Br J Cancer. 1999. 80:1020–1025.
Article15. Ranganathan S, Benetatos CA, Colarusso PJ, Dexter DW, Hudes GR. Altered beta-tubulin isotype expression in paclitaxel-resistant human prostate carcinoma cells. Br J Cancer. 1998. 77:562–566.
Article16. Urano N, Fujiwara Y, Doki Y, Kim SJ, Miyoshi Y, Noguchi S, et al. Clinical significance of class III beta-tubulin expression and its predictive value for resistance to docetaxel-based chemotherapy in gastric cancer. Int J Oncol. 2006. 28:375–381.17. Otrock ZK, Hatoum HA, Awada AH, Ishak RS, Shamseddine AI. Hypoxia-inducible factor in cancer angiogenesis: structure, regulation and clinical perspectives. Crit Rev Oncol Hematol. 2009. 70:93–102.
Article18. Calvani M, Trisciuoglio D, Bergamaschi C, Shoemaker RH, Melillo G. Differential involvement of vascular endothelial growth factor in the survival of hypoxic colon cancer cells. Cancer Res. 2008. 68:285–291.
Article19. Kuwai T, Kitadai Y, Tanaka S, Onogawa S, Matsutani N, Kaio E, et al. Expression of hypoxia-inducible factor-1alpha is associated with tumor vascularization in human colorectal carcinoma. Int J Cancer. 2003. 105:176–181.
Article20. Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003. 9:677–684.
Article21. Raspaglio G, Filippetti F, Prislei S, Penci R, De Maria I, Cicchillitti L, et al. Hypoxia induces class III beta-tubulin gene expression by HIF-1alpha binding to its 3' flanking region. Gene. 2008. 409:100–108.
Article22. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004. 350:2335–2342.
Article23. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007. 357:2666–2676.
Article24. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006. 355:2542–2550.
Article25. Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011. 29:3968–3976.
Article26. Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008. 8:942–956.
Article27. Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005. 65:671–680.28. Gille J, Heidenreich R, Pinter A, Schmitz J, Boehme B, Hicklin DJ, et al. Simultaneous blockade of VEGFR-1 and VEGFR-2 activation is necessary to efficiently inhibit experimental melanoma growth and metastasis formation. Int J Cancer. 2007. 120:1899–1908.
Article29. Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001. 7:1194–1201.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation of Vascular Endothelial Growth Factor-D Expression and VEGFR-3-Positive Vessel Density with Lymph Node Metastasis in Gastric Carcinoma
- Concomitant inhibition of epidermal growth factor and vascular endothelial growth factor receptor tyrosine kinases in oral squamous cell carcinoma
- Soluble and Membranous Vascular Endothelial Growth Factor Receptor-2 in Pregnancies Complicated by Pre-Eclampsia
- Targeting Receptor Tyrosine Kinase on Endothelial Cells in an Orthotopic Tumor Model of Oral Squamous Cell Carcinorma
- Inhibition of Lymphatic Endothelial Growth Factor Receptor in a Murine Model of Oral Squamous Cell Carcinoma