Yonsei Med J.
2009 Oct;50(5):656-666. 10.3349/ymj.2009.50.5.656.
Soluble and Membranous Vascular Endothelial Growth Factor Receptor-2 in Pregnancies Complicated by Pre-Eclampsia
- Affiliations
-
- 1Department of Anatomy, VMMC & Safdarjang Hospital, New Delhi, India. richa.trpth@gmail.com
- 2Department of Biochemistry, All India Institute of Medical Sciences, India.
- 3Institute of Pathology, Safdarjang Hospital, New Delhi, India.
- 4Department of Obstetrics and Gynaecology, VMMC & Safdarjang Hospital, New Delhi, India.
- KMID: 1103819
- DOI: http://doi.org/10.3349/ymj.2009.50.5.656
Abstract
- PURPOSE
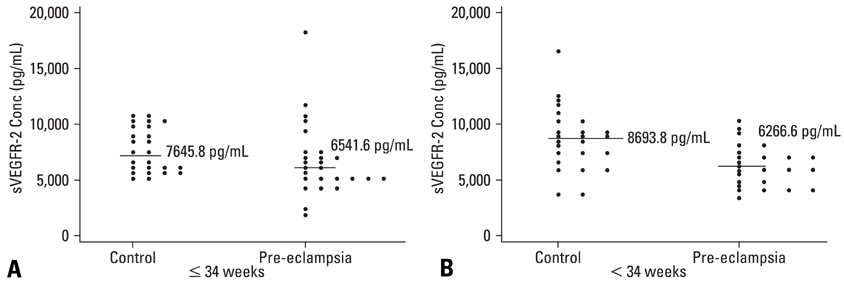
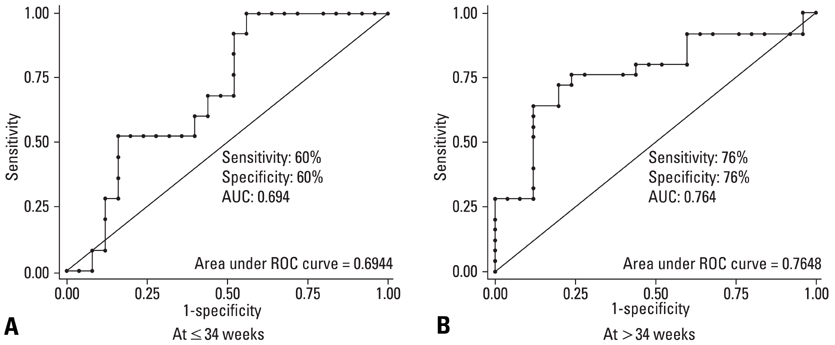
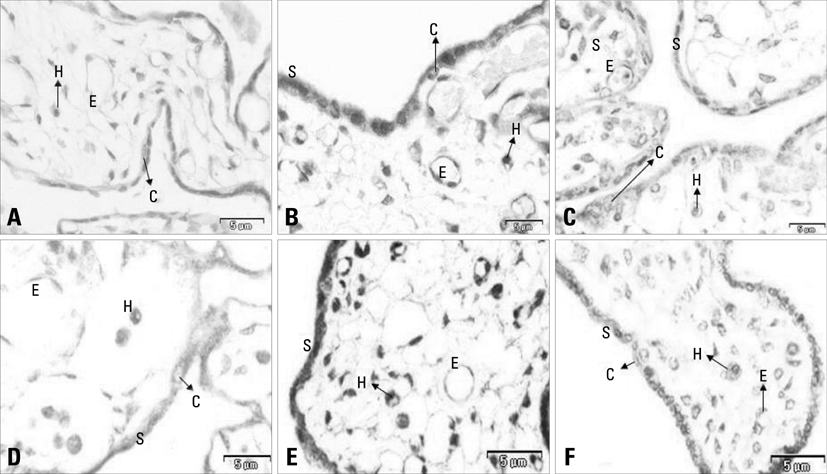
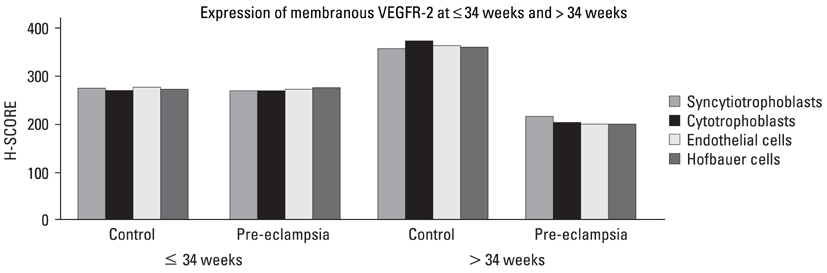
There is a paucity of information on the serum soluble vascular endothelial growth factor receptor-2 (sVEGFR-2) concentrations, membranous VEGFR-2 expression and the mechanism involved in their modulations during the clinical onset of pre-eclampsia. This cross-sectional study was conducted to evaluate the concentration of sVEGFR-2 in serum and to investigate the expression of membranous VEGFR-2 in placentae of pre-eclampsia group. MATERIALS AND METHODS: The serum levels of sVEGFR-2 (n = 120) and the expression of membranous VEGFR-2 in placentae (n = 100) were analysed at third trimester of pregnancy by enzyme linked immunosorbent assay (ELISA) and immunohistochemistry respectively. The diagnostic parameters of sensitivity, specificity and association of soluble and membranous VEGFR-2 in these patients were evaluated. RESULTS: The serum levels of sVEGFR-2 in pre-eclampsia patients were found to be significantly reduced (p = 0.01, p = 0.001) in early and late pre-eclamptic sub-groups as compared to their respective third trimester control sub-groups. Also, the receiver operating characteristic (ROC) curve analysis showed a cut-off value of 7350.4 pg/mL, higher sensitivity (76%) and specificity (76%) for sVEGFR-2 in late onset (> 34 weeks) pre-eclamptic group. Significant down-regulation of membranous VEGFR-2 immunoreactivity was observed in all the placental cells (p = 0.0001) at > 34 weeks preeclamptic group. CONCLUSION: The reduced serum levels of soluble VEGFR-2 and the down-regulated expression of membranous VEGFR-2 in the study group denoted abnormality in VEGF mediated placental function in all placental cells and thus VEGFR-2 may be a key factor, intimately associated with pre-eclampsia. This study shows the clinical utility of soluble and membranous VEGFR-2 in pre-eclampsia patients.
MeSH Terms
Figure
Reference
-
1. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in pregnancy. Am J Obstet Gynecol. 2000. 183:S1–S22.2. Talbert DG. Uterine flow velocity waveform shape as an indicator of maternal and placental development failure mechanisms: a model-based synthesizing approach. Ultrasound Obstet Gynecol. 1995. 6:261–271.
Article3. Guzin K, Tomruk S, Tuncay YA, Naki M, Sezginsoy S, Zemheri E, et al. The relation of increased uterine artery blood flow resistance and impaired trophoblast invasion in pre-eclamptic pregnancies. Arch Gynecol Obstet. 2005. 272:283–288.4. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003. 9:669–676.
Article5. Kondo K, Hiratsuka S, Subbalakshmi E, Matsushime H, Shibuya M. Genomic organization of the flt-1 gene encoding for vascular endothelial growth factor (VEGF) receptor-1 suggests an intimate evolutionary relationship between the 7-Ig and the 5-Ig tyrosine kinase receptors. Gene. 1998. 208:297–305.6. Hornig C, Behn T, Bartsch W, Yayon A, Weich HA. Detection and quantification of complexed and free soluble human vascular endothelial growth factor receptor-1 (sVEGFR-1) by ELISA. J Immunol Methods. 1999. 226:169–177.
Article7. Holash J, Davis S, Papadopoulos N, croll SD, HO L, Russell M, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002. 99:11393–11398.
Article8. Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995. 92:10457–10461.
Article9. Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992. 80:283–285.10. Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an antivascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003. 349:427–434.
Article11. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. Areversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996. 334:494–500.12. Tripathi R, Rath G, Jain A, Salhan S. Soluble and membranous vascular endothelial growth factor receptor-1 in pregnancies complicated by pre-eclampsia. Ann Anat. 2008. 190:477–489.
Article13. Ebos JM, Bocci G, Man S, Thorpe PE, Hicklin DJ, Zhou D, et al. A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma. Mol Cancer Res. 2004. 2:315–326.14. Agostini H, Boden K, Unsöld A, Martin G, Hansen L, Fiedler U, et al. A single local injection of recombinant VEGF receptor 2 but not of Tie2 inhibits retinal neovascularization in the mouse. Curr Eye Res. 2005. 30:249–257.
Article15. Robak E, Sysa-Jedrzejewska A, Robak T. Vascular endothelial growth factor and its soluble receptors VEGFR-1 and VEGFR-2 in the serum of patients with systemic lupus erythematosus. Mediators Inflamm. 2003. 12:293–298.16. Wierzbowska A, Robak T, Wrzesien-Kus A, Krawczynska A, Lech-Maranda E, Urbanska-Rys H. Circulating VEGF and its soluble receptors sVEGFR-1 and sVEGFR-2 in patients with acute leukemia. Eur Cytokine Netw. 2003. 14:149–153.17. Masuyama H, Suwaki N, Nakatsukasa H, Masumoto A, Tateishi Y, Hiramatrsu Y. Circulating angiogenic factors in preeclampsia, gestational proteinuria, and preeclampsia superimposed on chronic glomerulonephritis. Am J Obstet Gynecol. 2006. 194:551–556.
Article18. Demir R, Kayisli UA, Seval Y, Celik-Ozenci C, Korgun ET, Demir-Weusten AY, et al. Sequential expression of VEGF and its receptors in human placental villi during very early pregnancy: differences between placental vasculogenesis and angiogenesis. Placenta. 2004. 25:560–572.
Article19. Tas F, Duranyildiz D, Oguz H, Camlica H, Yasasever V, Topuz E. Circulating serum levels of angiogenic factors and vascular endothelial growth factor receptors 1 and 2 in melanoma patients. Melanoma Res. 2006. 16:405–411.
Article20. Cortelezzi A, Fracchiolla NS, Mazzeo LM, Silvestris I, Pomati M, Somalvico F, et al. Endothelial precursors and mature endothelial cells are increased in the peripheral blood of myelodysplastic syndromes. Leuk Lymphoma. 2005. 46:1345–1351.
Article21. Aref S, El Sherbiny M, Goda T, Fouda M, Al Askalany H, Abdalla D. Soluble VEGF/sFLt1 ratio is an independent predictor of AML patient out come. Hematology. 2005. 10:131–134.
Article22. Faderl S, Do KA, Johnson MM, Keating M, O'brien S, Jillani I, et al. Angiogenic factors may have a different prognostic role in adult acute lymphoblastic leukemia. Blood. 2005. 106:4303–4307.
Article23. Hu Q, Dey AL, Yang Y, Shen Y, Jilani IB, Estey EH, et al. Soluble vascular endothelial growth factor receptor 1, and not receptor 2, is an independent prognostic factor in acute myeloid leukemia and myelodysplastic syndromes. Cancer. 2004. 100:1884–1891.
Article24. Chaiworapongsa T, Romero R, Gotsch F, Espinoza J, Nien JK, Goncalves L, et al. Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J Matern Fetal Neonatal Med. 2008. 21:41–52.
Article25. Wen Y, Edelman JL, Kang T, Zeng N, Sachs G. Two functional forms of vascular endothelial growth factor receptor-2/Flk-1 mRNA are expressed in normal rat retina. J Biol Chem. 1998. 273:2090–2097.
Article26. Roeckl W, Hecht D, Sztajer H, Waltenberger J, Yayon A, Weich HA. Differential binding characteristics and cellular inhibition by soluble VEGF receptors 1 and 2. Exp Cell Res. 1998. 24:161–170.
Article27. Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase-1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003. 111:649–658.
Article28. Wallner W, Sengenberger R, Strick R, Strissel PL, Meurer B, Beckmann MW, et al. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clin Sci (Lond). 2007. 112:51–57.
Article29. Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004. 145:4838–4845.
Article30. Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001. 114(Pt 5):853–865.
Article31. Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993. 90:10705–10709.
Article32. Reuvekamp A, Velsing-Aarts FV, Poulina IE, Capello JJ, Duits AJ. Selective deficit of angiogenic growth factors characterises pregnancies complicated by pre-eclampsia. Br J Obstet Gynaecol. 1999. 106:1019–1022.
Article33. Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem. 1997. 272:23659–23667.
Article34. Kim SY, Park SY, Kim JW, Kim YM, Yang JH, Kim MY, et al. Circulating endothelial progenitor cells, plasma VEGF, VEGFR-1 andVEGFR-2 levels in pre-eclampsia. Am J Obstet Gynecol. 2005. 193:S74.35. Khan SS, Solomon MA, McCoy JP Jr. Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytometry B Clin Cytom. 2005. 64:1–8.
Article36. Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005. 353:999–1007.
Article37. Sugawara J, Mitsui-Saito M, Hayashi C, Hoshiai T, Senoo M, Chisaka H, et al. Decrease and senescence of endothelial progenitor cells in patients with preeclampsia. J Clin Endocrinol Metab. 2005. 90:5329–5332.
Article38. Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005. 366:1797–1803.
Article39. Smith GD, Sterne J, Tynelius P, Lawlor DA, Rasmussen F. Birth weight of offspring and subsequent cardiovascular mortality of the parents. Epidemiology. 2005. 16:563–569.
Article40. Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006. 39:469–478.
Article41. Takahashi H, Hattori S, Iwamatsu A, Takizawa H, Shibuya M. A novel snake venom vascular endothelial growth factor (VEGF) predominantly induces vascular permeability through preferential signaling via VEGF receptor-1. J Biol Chem. 2004. 279:46304–46314.
Article42. Cooper JC, Sharkey AM, McLaren J, Charnock-Jones DS, Smith SK. Localization of vascular endothelial growth factor and its receptor, flt, in human placenta and deciduas by immunohistochemistry. J Reprod Fertil. 1995. 105:205–213.
Article43. Clark DE, Smith SK, Sharkey AM, Charnock-Jones DS. Localization of VEGF and expression of its receptors flt and KDR in human placenta throughout pregnancy. Hum Reprod. 1996. 11:1090–1098.
Article44. Vuckovic M, Ponting J, Terman BI, Niketie V, Seif MW, Kumar S. Expression of the vascular endothelial growth factor receptor, KDR, in human placenta. J Anat. 1996. 188(Pt 2):361–366.45. Shibuya M. Structure and dual function of vascular endothelial growth factor receptor-1 (Flt-1). Int J Biochem Cell Biol. 2001. 33:409–420.
Article46. Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(x) cells identifies a population of functional endothelial precursors. Blood. 2000. 95:952–958.
Article47. Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002. 160:1405–1423.
Article48. Helske S, Vuorela P, Carpén O, Hornig C, Weich H, Halmesmäki E. Expression of vascular endothelial growth factor receptors 1, 2 and 3 in placentas from normal and complicated pregnancies. Mol Hum Reprod. 2001. 7:205–210.
Article49. Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, et al. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003. 88:5555–5563.
Article50. Rajakumar A, Brandon HM, Daftary A, Ness R, Conrad KP. Evidence for the functional activity of hypoxiainducible transcription factors overexpressed in preeclamptic placentae. Placenta. 2004. 25:763–769.
Article51. Giraudo E, Primo L, Audero E, Gerber HP, Koolwijk P, Soker S, et al. Tumor necrosis factor-alpha regulates expression of vascular endothelial growth factor receptor-2 and of its coreceptor neuropilin-1 in human vascular endothelial cells. J Biol Chem. 1998. 273:22128–22135.
Article52. Shen BQ, Lee DY, Gerber HP, Keyt BA, Ferrara N, Zioncheck TF. Homologous up-regulation of KDR/Flk-1 receptor expression by vascular endothelial growth factor in vitro. J Biol Chem. 1998. 273:29979–29985.
Article53. Detmar M, Brown LF, Berse B, Jackman RW, Elicker BM, Dvorak HF, et al. Hypoxia regulates the expression of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) and its receptors in human skin. J Invest Dermatol. 1997. 108:263–268.
Article54. Elvert G, Kappel A, Heidenreich R, Englmeier U, Lanz S, Acker T, et al. Cooperative interaction of hypoxia-inducible factor-2alpha (HIF-2alpha) and Ets-1 in the transcriptional activation of vascular endothelial growth factor receptor-2 (Flk-1). J Biol Chem. 2003. 278:7520–7530.
Article55. Roberts JM, Lain KY. Recent insights into the pathogenesis of pre-eclampsia. Placenta. 2002. 23:359–372.
Article56. Mukherjee S, Tessema M, Wandinger-Ness A. Vesicular trafficking of tyrosine kinase receptors and associated proteins in the regulation of signaling and vascular function. Circ Res. 2006. 98:743–756.
Article57. Singh AJ, Meyer RD, Band H, Rahimi N. The carboxyl terminus of VEGFR-2 is required for PKC-mediated down-regulation. Mol Biol Cell. 2005. 16:2106–2118.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Levels of Circulating Vascular Endothelial Growth Factor and Soluble Flt-1 in Pregnancies Complicated by Preeclampsia
- Maternal Preeclampsia and Bronchopulmonary Dysplasia
- The Expression of Vascular Endothelial Growth Factor, Platelet-Derived Growth Factor and Intercellular Adhesion Molecule in Severe Preeclamptic Placenta
- Cord Blood Soluble fms-Like Tyrosine Kinase 1 and Placental Growth Factor in Preterm Infants with Maternal Preeclampsia
- Clinical usefulness of soluble fms-like tyrosine kinase 1, soluble endoglin and placental growth factor in Korean women with preeclampsia