Yonsei Med J.
2008 Apr;49(2):295-300. 10.3349/ymj.2008.49.2.295.
The Effect of HIF-1alpha siRNA on Growth and Chemosensitivity of Mia-paca Cell Line
- Affiliations
-
- 1Department of Surgery, Winship Cancer Institute, Emory University, Atlanta, GA, USA. wonkkang@catholic.ac.kr
- 2Department of Surgery, The Catholic University of Korea, Seoul, Korea.
- KMID: 1084504
- DOI: http://doi.org/10.3349/ymj.2008.49.2.295
Abstract
- PURPOSE
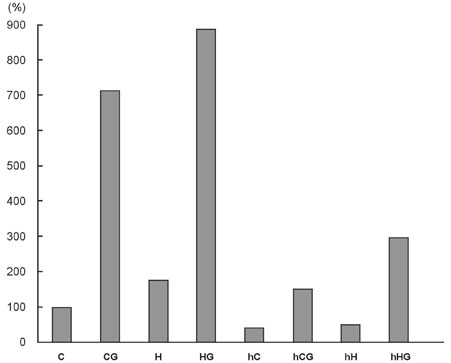
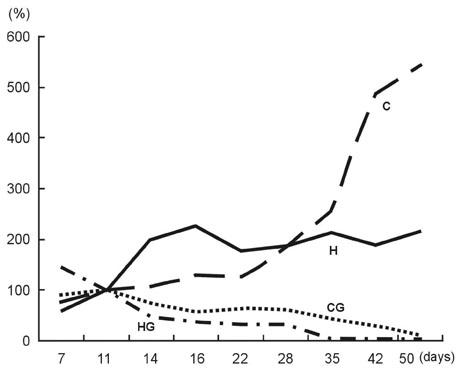
Hypoxia-inducible factor-1alpha (HIF-1alpha) primarily mediates the hypoxic response. HIF-1alpha induction by various stimuli contributes to cell proliferation and survival. To investigate the effect of HIF-1alpha, we used small interfering RNA (siRNA), and expected that cell apoptosis and sensitivity to chemotherapeutic drug increase, when we blocked the HIF-1alpha gene. Thus we performed in vitro and in vivo experiment to clarify the effect of hypoxia-inducible factor-1alpha on tumor growth. MATERIALS AND METHODS: We made control and HIF-1alpha siRNA using vector plasmid and then transfected Mia-paca cell lines with these RNAs. After selection with geneticin, two new cell lines were made, confirmed via immunoblotting. After treating with gemcitabine, each cell line was assayed to confirm the effect of HIF-1alpha siRNA using the cell proliferation assay and capase-3 assay. And then in vivo study was performed using female athymic nude mice. After subcutaneously injecting each new cell lines, intraperitoneal gemicitabine chemotherapy was performed for 3 weeks. During that period, we analyzed the difference of tumor growth rate. RESULTS: The tumor growth of HIF-1alpha siRNA-transfected group was slower than that of the control group both in vitro and in vivo experiment. CONCLUSION: The suppression of HIF-1alpha results in decrease of cell proliferation and increase of chemosensitivity of pancreatic cancer cell line. Therefore, targeting the HIF-1alpha may be useful treatment modality for some pancreatic cancers.
MeSH Terms
-
Animals
Antimetabolites, Antineoplastic/pharmacology
Blotting, Western
Caspase 3/metabolism
Cell Hypoxia
Cell Line, Tumor
*Cell Proliferation
Cell Survival/drug effects
Deoxycytidine/analogs & derivatives/pharmacology
Female
Humans
Hypoxia-Inducible Factor 1, alpha Subunit/*genetics/physiology
Mice
*RNA Interference
RNA, Small Interfering/*genetics
Random Allocation
Transfection
Figure
Reference
-
1. Déry MA, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol. 2005. 37:535–540.
Article2. Bae SH, Jeong JW, Park JA, Kim SH, Bae MK, Choi SJ, et al. Sumoylation increases HIF-1alpha stability and its transcriptional activity. Biochem Biophys Res Commun. 2004. 324:394–400.3. Zagórska A, Dulak J. HIF-1: the knowns and unknowns of hypoxia sensing. Acta Biochim Pol. 2004. 51:563–585.
Article4. Dorsett Y, Tuschl T. siRNAs: applications in functional genomics and potential as therapeutics. Nat Rev Drug Discov. 2004. 3:318–329.
Article5. Ryther RC, Flynt AS, Phillips JA 3rd, Patton JG. siRNA therapeutics: big potential from small RNAs. Gene Ther. 2005. 12:5–11.
Article6. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007. 57:43–66.
Article7. Bold RJ, Chandra J, McConkey DJ. Gemcitabine-induced programmed cell death (apoptosis) of human pancreatic carcinoma is determined by Bcl-2 content. Ann Surg Oncol. 1999. 6:279–285.
Article8. Fujiwara S, Nakagawa K, Harada H, Nagato S, Furukawa K, Teraoka M, et al. Silencing hypoxia-inducible factor-1alpha inhibits cell migration and invasion under hypoxic environment in malignant gliomas. Int J Oncol. 2007. 30:793–802.9. Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002. 296:1655–1657.
Article10. Debatin KM. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol Immunother. 2004. 53:153–159.
Article11. Karagiannis TC, El-Osta A. siRNAs: mechanism of RNA interference, in vivo and potential clinical applications. Cancer Biol Ther. 2004. 3:1069–1074.
Article12. Chang HS, Lin CH, Chen YC, Yu WC. Using siRNA technique to generate transgenic animals with spatiotemporal and conditional gene knockdown. Am J Pathol. 2004. 165:1535–1541.
Article13. Hsieh AC, Bo R, Manola J, Vazquez F, Bare O, Khvorova A, et al. A library of siRNA duplexes targeting the phosphoinositide 3-kinase pathway: determinants of gene silencing for use in cell-based screens. Nucleic Acids Res. 2004. 32:893–901.
Article14. Zhao Q, Du J, Gu H, Teng X, Zhang Q, Qin H, et al. Effects of YC-1 on hypoxia-inducible factor 1-driven transcription activity, cell proliferative vitality, and apoptosis in hypoxic human pancreatic cancer cells. Pancreas. 2007. 34:242–247.
Article15. Yang L, Cao Z, Li F, Post DE, Van Meir EG, Zhong H, et al. Tumor-specific gene expression using the survivin promoter is further increased by hypoxia. Gene Ther. 2004. 11:1215–1223.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of hypoxia inducible factors-1alpha on autophagy and invasion of trophoblasts
- The Relationship between Expression of Hypoxia Inducible Factor-1alpha or Vascular Endothelial Growth Factor and Histopathological Characteristics in Human Renal Cell Carcinoma
- The Expression of Hypoxia Inducible Factor-1alpha by Desferrioxamine Induces Radioresistance in Mouse Hepatoma Cell Line
- The Relationship between Expression of Hypoxia Inducible Factor-1alpha or Vascular Endothelial Growth Factor and Histopathological Characteristics in Human Transitional Bladder Cancer
- The Effect of Epigallocatechin-3-gallate on HIF-1alpha and VEGF in Human Lung Cancer Cell Line