Tuberc Respir Dis.
2009 Mar;66(3):178-185. 10.4046/trd.2009.66.3.178.
The Effect of Epigallocatechin-3-gallate on HIF-1alpha and VEGF in Human Lung Cancer Cell Line
- Affiliations
-
- 1Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea. iwpark@cau.ac.kr
- 2Department of Microbiology, Chung-Ang University College of Medicine, Seoul, Korea.
- KMID: 1846377
- DOI: http://doi.org/10.4046/trd.2009.66.3.178
Abstract
-
BACKGROUND: Epigallocatechin-3-gallate (EGCG) is the major catechin in green tea, and has shown antiproliferative, antiangiogenic, antimetastatic and cell cycle pertubation activity in various tumor models. Hypoxia can be induced because angiogenesis is insufficient for highly proliferating cancer. Hypoxia-inducible factor-1alpha (HIF-1alpha) and its downstream target, vascular endothelial growth factor (VEGF), are important for angiogenesis, tumor growth and metastasis. The aim of this study was to determine how hypoxia could cause changes in the cellular phenomena and microenvironment in a non-small cell culture system and to examine the effects of EGCG on a HIF-1alpha and VEGF in A549 cell line.
METHODS
A549 cells, a non-small cell lung cancer cell line, were cultured with DMEM and 10% fetal bovine serum. A decrease in oxygen tension was induced using a hypoxia microchamber and a CO2-N2 gas mixture. Gas analysis and a MTT assay were performed. The A549 cells were treated with EGCG (0, 12.5, 25, 50 micromol/L), and then examined by real-time-PCR analysis of HIF-1alpha, VEGF, and beta-actin mRNA.
RESULTS
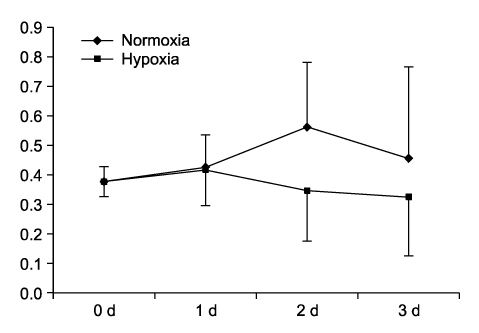
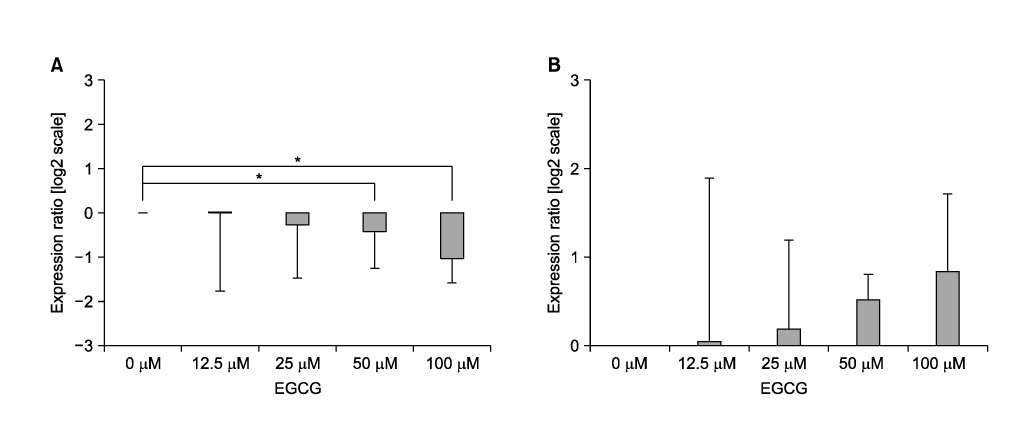
Hypoxia reduced the proliferation of A549 cells from normoxic conditions. EGCG inhibited HIF-1alpha transcription in A549 cells in a dose-dependent manner. Compared to HIF-1alpha, VEGF was not inhibited by EGCG.
CONCLUSION
HIF-1alpha can be inhibited by EGCG. This suggests that targeting HIF-1alpha with a EGCG treatment may have therapeutic potential in non-small cell lung cancers.
Keyword
MeSH Terms
Figure
Reference
-
1. O'Byrne KJ, Dalgleish AG, Browning MJ, Steward WP, Harris AL. The relationship between angiogenesis and the immune response in carcinogenesis and the progression of malignant disease. Eur J Cancer. 2000. 36:151–169.2. Brown JM. The hypoxic cell: a target for selective cancer therapy--eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1999. 59:5863–5870.3. Rofstad EK, Danielsen T. Hypoxia-induced metastasis of human melanoma cells: involvement of vascular endothelial growth factor-mediated angiogenesis. Br J Cancer. 1999. 80:1697–1707.4. Rofstad EK, Mathiesen B, Henriksen K, Kindem K, Galappathi K. The tumor bed effect: increased metastatic dissemination from hypoxia-induced up-regulation of metastasis-promoting gene products. Cancer Res. 2005. 65:2387–2396.5. Büchler P, Reber HA, Lavey RS, Tomlinson J, Büchler MW, Friess H, et al. Tumor hypoxia correlates with metastatic tumor growth of pancreatic cancer in an orthotopic murine model. J Surg Res. 2004. 120:295–303.6. Cangul H. Hypoxia upregulates the expression of the NDRG1 gene leading to its overexpression in various human cancers. BMC Genet. 2004. 5:27.7. Song X, Liu X, Chi W, Liu Y, Wei L, Wang X, et al. Hypoxia-induced resistance to cisplatin and doxorubicin in non-small cell lung cancer is inhibited by silencing of HIF-1alpha gene. Cancer Chemother Pharmacol. 2006. 58:776–784.8. Koukourakis MI, Giatromanolaki A, Sivridis E, Fezoulidis I. Cancer vascularization: implications in radiotherapy? Int J Radiat Oncol Biol Phys. 2000. 48:545–553.9. Swinson DE, O'Byrne KJ. Interactions between hypoxia and epidermal growth factor receptor in non-small-cell lung cancer. Clin Lung Cancer. 2006. 7:250–256.10. Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000. 88:1474–1480.11. Wang T, Niki T, Goto A, Ota S, Morikawa T, Nakamura Y, et al. Hypoxia increases the motility of lung adenocarcinoma cell line A549 via activation of the epidermal growth factor receptor pathway. Cancer Sci. 2007. 98:506–511.12. Koshikawa N, Takenaga K, Tagawa M, Sakiyama S. Therapeutic efficacy of the suicide gene driven by the promoter of vascular endothelial growth factor gene against hypoxic tumor cells. Cancer Res. 2000. 60:2936–2941.13. Li L, Yu J, Xing L, Ma K, Zhu H, Guo H, et al. Serial hypoxia imaging with 99mTc-HL91 SPECT to predict radiotherapy response in nonsmall cell lung cancer. Am J Clin Oncol. 2006. 29:628–633.14. Shin JW, Jeon EJ, Kwak HW, Song JH, Lee YW, Jeong JW, et al. Microenvironments and cellular proliferation affected by oxygen concentration in non-small cell lung cancer cell line. Tuberc Respir Dis. 2007. 63:242–250.15. Kobayashi S, Conforti L, Pun RY, Millhorn DE. Adenosine modulates hypoxia-induced responses in rat PC12 cells via the A2A receptor. J Physiol. 1998. 508:95–107.16. Ziemer LS, Lee WM, Vinogradov SA, Sehgal C, Wilson DF. Oxygen distribution in murine tumors: characterization using oxygen-dependent quenching of phosphorescence. J Appl Physiol. 2005. 98:1503–1510.17. Mairbäurl H, Wodopia R, Eckes S, Schulz S, Bärtsch P. Impairment of cation transport in A549 cells and rat alveolar epithelial cells by hypoxia. Am J Physiol. 1997. 273:L797–L806.18. O'Kelly I, Peers C, Kemp PJ. O2-sensitive K+ channels in neuroepithelial body-derived small cell carcinoma cells of the human lung. Am J Physiol. 1998. 275:L709–L716.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Relationship between Expression of Hypoxia Inducible Factor-1alpha or Vascular Endothelial Growth Factor and Histopathological Characteristics in Human Transitional Bladder Cancer
- Immunohistochemical Expression and Prognostic Value of VEGF, HIF-1alpha, EGFR in Non-Small Cell Lung Cancer
- The Relationship between Expression of Hypoxia Inducible Factor-1alpha or Vascular Endothelial Growth Factor and Histopathological Characteristics in Human Renal Cell Carcinoma
- Expression of Hypoxia-inducible Factor-1alpha in Non-small Cell Lung Cancer: Relationship to Prognosis and Tumor Biomarkers
- The Effect of HIF-1alpha siRNA on Growth and Chemosensitivity of Mia-paca Cell Line